NNI NewsPaper 1/2018 - A Summary of the latest scientific news: Heros in the gut
Research in recent years has demonstrated that HMOs play a contributory role in protecting infants from disease in developing countries
Significance of the Microbiome
Author:
Dr. med. Mike Poßner
Medical Director Europe
Nestlé Nutrition Institute
Editorial
Dear Readers,
For healthy development and growth in childhood, the intestinal flora, or microbiome, plays a key role. The particular makeup of breast milk provides the optimum nutrition for the newly born child and a support for the microbiome. This contains an abundance of positive sub- stances.
Current interest has been focusing on the so-called human milk oligosaccharides (HMO) as a centre of particular attention, which represents an essential part of the content of breast milk. The importance of these components has already been known to researchers for some time, but it has not been possible until recently to enrich infant formulae with selected and particularly important HMOs. These HMOs are the same as those found in breast milk. They have been positively evaluated both in Europe and in the United States („Novel Food“ status by the EFSA, GRAS status by the FDA). The first results of these studies look very promising. Learn more about this exciting topic on the following pages!
Wonderworld of the microbiome
“An advance in research, as significant as the moon landing!” That is how otherwise very down-to-earth and serious experts enthuse about the most recent developments in food science. And not without good reason, because within just a short period of time the conditions have been created which open up a new era in infant nutrition. The secret is called HMO.
Ten years ago the microbiome – which used to be referred to as the intestinal flora – was a field of study which was only understood by a few specialists in microbiology. In the meantime, however, this has become a booming area of research. It is becoming increasingly evident that this extraordinarily complex area can be attributed a decisive role in development, well-being and long-term health. Even feelings and behaviour seem to be influenced by it. The microbiome is increasingly being recognised as an organ in its own right – a quite special organ, as it changes throughout the course of a lifetime – and we can change it, as well!
Millions of good friends
The dimensions alone are impressive! Be- cause the microbiome includes bacteria, fungi, viruses, and the metabolites produced by them in the body. The intestinal microbiome – i.e. all of the microbes that inhabit the human gut, give rise to some amazing numbers:
Intestinal flora of adults:
- 100 trillion (1014) bacteria
- 10 x more than all cells in the body
- About 36,000 different species
- Approx. 1–2 kg of biomass
- Intestinal microbiome encompasses about 3.3 million genes
- Roughly 150 times more than the human genome
This microbiome obviously does not exist in a random form, there are preferred combinations of bacteria, the so-called enterotypes, which are apparently related to dietary habits.
Is the gut a second brain?
The relationship to health is particularly interesting. Because more and more diseases, which are not causally related to the intestines, seem to be significantly influenced by the state of the microbiome, including respiratory diseases, obesity, type 2 diabetes, allergies, and asthma. There is even evidence that autism, depression or chronic pain are due to disorders of the gut-brain axis. As a consequence of these new findings, the current Rome IV guide- lines for functional gastro-intestinal disorders ( FGID) recommend in future to use the wording “Disorders of Gut-Brain Inter- action” ( DGBI).
Some are even now saying that the intestine is a “second brain”, which controls our thoughts and (sub) conscious similarly to the brain. It is therefore an inescapable conclusion that a well balanced intestinal microbiome has a significant influence on health, and therefore nutrition is an important factor ( Fig. 1).
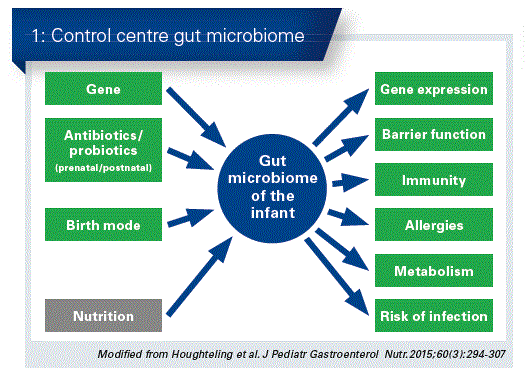
Breast milk is the best
In this context, the composition of breast milk plays a decisive role. Because in this way, the infantile organism is introduced to the most important components of a healthy intestinal flora. Breast milk provides the most important „probiotic“ bifidus and lactobacillus bacteria, whose positive effect has been well-known for a long time. With 800 ml breast milk per day, a baby ingests about 1 million bacteria, which includes bifidus and lactobacillus bacteria. Many factors contribute to the bifidogenic effect of breast milk.
All-round talent HMO
Amongst the many components with a protective effect, the human milk oligosaccharides (HMOs) play a decisive role. HMOs occur solely in breast milk. After the milk sugar lactose and the milk fats, milk oligosaccharides represent the third largest component group in breast milk (Fig. 2).
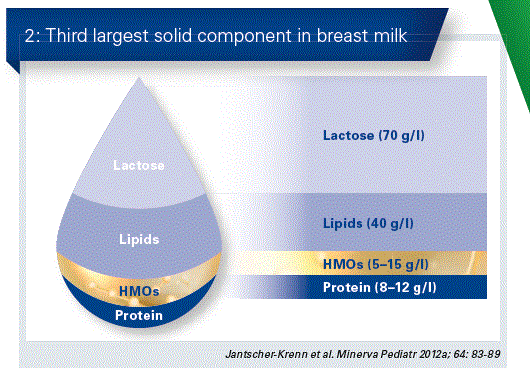
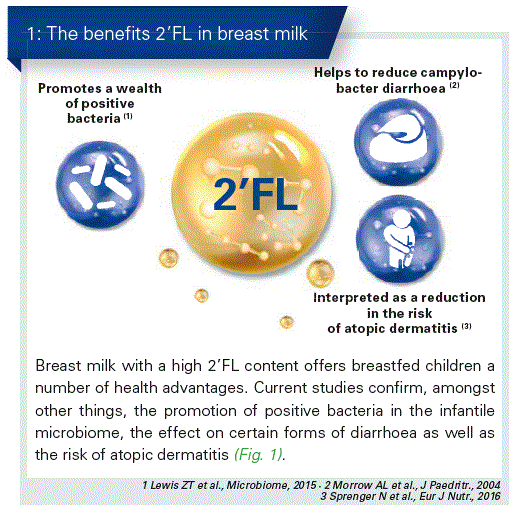
HMOs occur in a great diversity of different structures and amounts. The individual composition depends upon various factors. The quantitative largest compound is in this case 2’-fucosyllactose (2’FL), which accounts in most cases for almost one third of the entire HMO content. (Fig. 3). 2’FL has a particularly positive effect on the microbiome of breast- fed children.
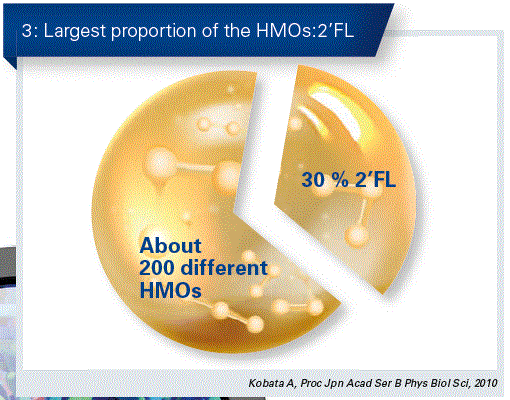
For more than one hundred years, scientists and paediatricians have been fascinated about these important components in breast milk, both from their diversity as well as from their benefit on the infantile organism. More recently, technological advances in the manufacture of individual HMOs have made it possible to study these also in placebo-controlled and randomised clinical trials.
Human milk oligosaccharides
Complex sugars with effect
Human milk oligosaccharides (HMOs) are an essential nutritional component for breastfed children. A lack of these components is the most important difference between breast milk and infant formula.
Human milk oligosaccharides have a special structure, they occur in a large variety of forms and make up the third largest component in breast milk. HMOs are relatively simple in their structure, but ultimately all of them are based on five basic substances. Almost all HMOs are based on lactose, which is modified in the mammary gland by the deposition of monosaccharides such as fucose, N-acetylglu- cosamine, and/or sialinic acid. In this way, complex structures with very specific links are formed, which is the basis for the multi-functionality of HMO (Fig. 1).
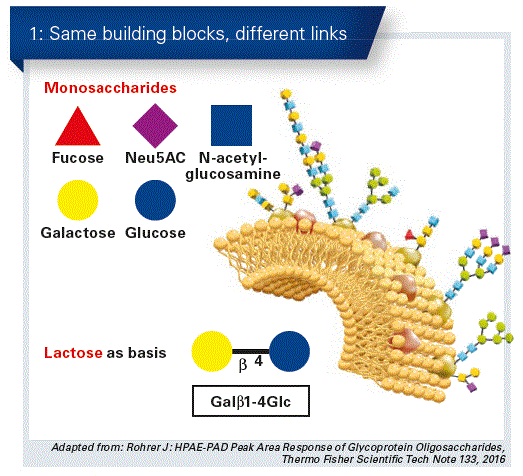
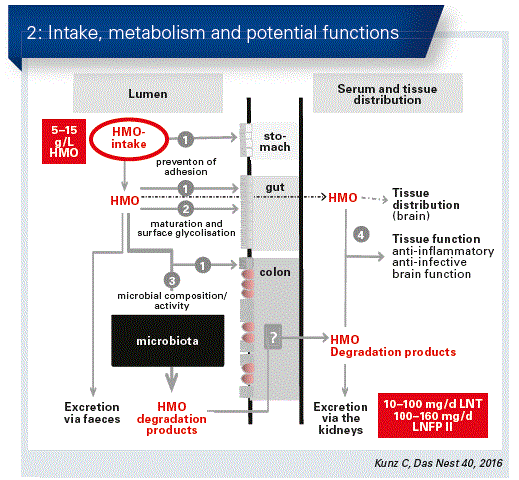
The total concentration of HMOs in mature breast milk is between 10 and 15 g/L – in colostrum this may be even higher. For most mothers, 2’FL makes up the largest proportion with about 30 percent.
Bifidogenic effect
If the microbiotic composition of the gut of breastfed infants is analysed, normally there is a distinct prevalence of bifidus bacteria and a smaller proportion of potentially pathogenic bacteria. By stimulation of bifidus bacteria using different HMOs, the introduction of pathogenic substances can be prevented, as György was able to establish already in 1955. More recent data allow the conclusion to be made that apparently certain bacterial sub-strains possess the ability to utilise the HMOs and/or different forms of HMOs (Fig. 2).
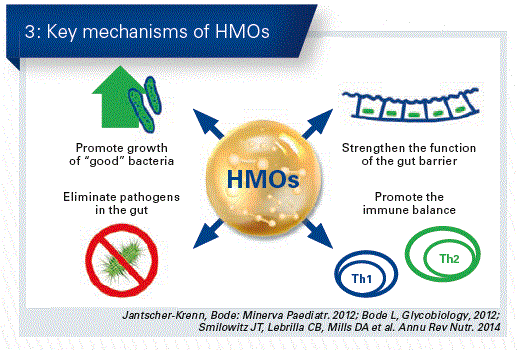
In this way, it becomes progressively clearer that HMOs are really great all-rounders for a healthy development (Fig. 3).
- HMOs promote the growth of specific bifdus bacteria in the infantile gut
- HMOs are the “decoy” for pathogens. They adhere to the HMOs and are excreted together with them in the stools.
- HMOs strengthen the gut barrier function, they prevent adhesion of potential pathogens to the intestinal cells by “reprogramming” them.
- HMOs have an immune regulatory effect, they contribute to the development of balanced Th1/Th2-immune responses.
Interview
HMO: More than just prebiotics
In more recent years, it has become normal practice to add certain carbohydrates such as galacto- (GOS) and fructo-oligosaccharides (FOS) to infant formula, as they promise to have a positive effect on the development of the infantile microbiome. They are not the same as human oligosaccharides (HMOs), however. These have in their mechanism of action a more far reaching action than GOS/FOS, however.
A significant difference is to be found in that FOS does not occur in breast milk at all and GOS is only present in extremely low amounts. Although these prebiotics also promote the colonisation of bifidogenic bacteria in the gut of neonates and small children, their effect is altogether considerably more limited than in the case of HMOs. This is due to the differences in structure:
HMOs have more complex structures and components, which do not arise in FOS/ GOS.
- FOS have two components and a kind of link as simple as Lego building blocks
- GOS also have two components, however, different forms of linkage
- HMOs consist of at least three components and have a multitude of different linkages
Many functions of HMOs are dependent upon structure. For this reason, FOS/GOS are not able to achieve the mechanism of action of the HMOs.
Prebiotics
- Have a less specific action on the gut microbiome
- Are not able to “lure” and eliminate pathogens
- Are not able to directly strengthen the gut barrier function
- Have no direct effect on the immune balance
First HMOs offer the positive characteristics of breast milk and make it possible to offer a comprehensive protective effect to the infantile flora of the gut.
On the trail of the oligosaccharides
The first description of micro-organisms such as lactobacillus and bifidobacteria was already made around the year 1900. Even at that time, there were the first indications that the different composition of faeces of breastfed and bottle-fed children was associated with the different carbohydrates. Then at the beginning of the 1930’s, the first individual breast milk oligosaccharides were characterised.
A quantum leap in HMO research was achieved in the 1950’s when the paediatrician Paul György and the chemist Richard Kuhn were able to demonstrate that the growth factor for Lactobacillus bifidus (later classified as Bifidobacterium bifidus) exists in breast milk as oligosaccharides
In the following years, it was possible to discover and classify more than a dozen HMOs. Since the turn of the millennium, impressive advances have been made in the development of methods for analysing the structure in detail, even with extremely low amounts of milk. At the same time, it was also possible to produce larger amounts of individual HMOs using chemical and biotechnical means.
After the prebiotic effect had been initially investigated, the focus was also increasingly put on other health advantages which are associated with HMOs. It was now discussed how and which oligosaccharides could be added to infant’s milk in order to achieve similar positive effects such as HMOs in breast milk.
In 2012, the first clinical trial was carried out with HMO supplemented nutrition, after numerous in-vitro and animal studies had shown very promising results
2’FL in breast milk
2’FL – the content differs in breast milk
A cross-section study investigated the composition of milk of Chinese mothers living in three large cities in China, in order to determine the influence of secretory status, the breastfeeding time, the mode of giving birth and the place of birth. Samples taken from 446 mothers in different stages of lactation (90 each per phase) were investigated according to the content of 10 different HMOs.
Results
- Secretory status: Approximately 21% of samples showed a 2’-fucosyllactose level (2’-FL) which was less than the limit of detection, which corresponds to about the level of non-secretors in other countries. However, despite this 2’FL was present in all samples.
- Breastfeeding time: Of the factors which were investigated, only the stage of lactation had an influence on the composition of HMOs in breast milk.
- Place of birth: In contrast to previous studies, the geographical differences did not play any role. More than 90% of the mothers belonged to the Han clan, which possibly could explain this homogenous result.
Austin S et al.: Temporal Change of the Content of 10 Oligo- saccharides in the Milk of Chinese Urban Mothers. Nutrients 2016, 8, 346
Secretor status and infantile development
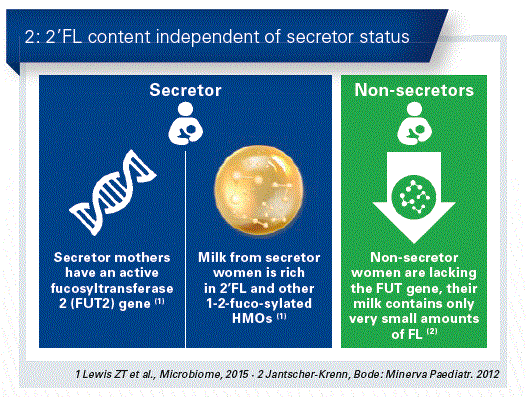
The HMO content of breast milk is not the same in all women, there are significant differences which are seen especially in the total content and the 2’FL values. In this respect, the genetic background is a decisive factor (Fig. 2).
A gene influences the enzyme fucosyltransferase 2 (FUT2), which has a particular effect on 2’FL. Whereas it accounts in most mothers for the largest proportion of the HMO structures (so-called secretors), in about 20% it is only slightly or even not present at all (“non-secretors”). Apparently, however, the Lewis blood groups also play a role in the amount and structure of the HMOs.
In an open monitoring study, milk was collected from 50 mothers after 30, 60 and 120 days postpartum, who had given birth to 25 male and/or female single children, respectively. On the 30th day following the start of breastfeeding, the milk samples and the respective children were grouped in clusters according to low (non-secretors) and high (secretors) amount of 2’FL concentrations in the milk.
Up until the 4th month there were no differences between the groups with regard to bodyweight, height, BMI and head circumference, independent of gender. The differences between the high and low 2’FL clusters obviously had no influence on the growth of the infant.
Sprenger N et al. (2017): Longitudinal change of selected human milk oligosaccharides and association to infants‘ growth, an ob- servatory, single center, longitudinal cohort study. PLoS ONE 12(2): e0171814.
“We are at the start of a new era in infant nutrition”
An interview with Prof. Clemens Kunz
Institute for Food Sciences, Justus-Liebig-University, Giessen
Prof. Kunz, you have been researching for many years in this area and are one of the most know-ledgable persons of the HMOs. Is this really a panacea, as the oligosaccharides are now appearing in all kinds of publications?
The advantages of the HMOs for infants are apparently very manifold, as we know from preclinical trials; however, this research is just beginning in the case of infants. Of course, one should not speak of a wonder drug, however the positive influence in particular on the microbiome of infants and on gastrointestinal disorders suggests this – this is what the results of the fi rst clinical trials in infants have suggested so far. However, despite the lengthy previous history of this research, we are still now only at the beginning of its practical application.
The presence of HMOs is considered the largest difference in the composition of breast milk in comparison with conventional infant formula. Can this gap be closed with supplementation?
Indeed, HMOs make the greatest difference in the composition between breast milk and infant formula. This is due to the fact that these oligosaccharides, which are typical for women’s breast milk, do not occur in cow’s milk, or only in traces. Also, prebiotics, which have been previously used to promote bifidobacteria in the gut microbiota, are not comparable with HMOs, although this is almost always carried out also in scientific studies. The increasing availability of individual HMOs in larger amounts means that we are at the beginning of a new era of research into infantile nutrition and in particular their influence on health. Although the above-mentioned gap has not been completely closed for cost reasons, I assume, however, that we will be able to do this with the main components.
Although there are a multitude of HMOs, are some of them of more particular significance than others?
HMOs show an extremely high structural diversity. 150-200 components have been identified up until now. Due to their complex composition, they are predestined for the multifunctionality which is being said about them. 5 to 7 individual components form the main group of HMOs; these also include 2’fucosyllactose (2’FL), which is currently playing a particular role in research. This 2´FL, and Lacto-N-neotetraose (LNnT), one component which likewise occurs in breast milk, are also among the first which are now being added to infant formula.
Is the mother a secretor or a non-secretor?
What are the consequences for the health of the infant? Milk from individual mothers can vary quite considerably. This depends, for example, on whether the milk originates from the first few days after giving birth or was collected at a later point in time. Significant deviations also depend upon the secretor and non-secretor status of the mother. The reason for this is a gene in the mammary gland which is responsible for an enzyme, namely fucosyltransferase 2 (FUT2). This enzyme modifies the basic structures of the HMOs. On the other hand, this also acts on the HMO pattern and also on the amount of neutral HMOs and hence on the entire amount of HMOs. For example, 2´FL is the main component in the so-called “secretor milk”, whereas in “non-secretor milk” this oligosaccharide is not even present. In Europe, about 80 % of mothers are so-called “secretors” and 20 % are non-secretors. It is extremely interesting in this respect that this secretor/non-secretor status is being very intensively discussed at the moment in connection with bacterial and viral diseases. It is well known, for example, that in the case of secretors the viability for noro virus infections is much higher than with non-secretors, whereas non-secretors have a higher risk for the development of Crohn’s disease.
So you are now increasingly demanding to have the results of in vitro and animal experiments verified in clinical trials?
In the meantime, we have a multitude of in vitro and animal trials, from which in part some rather daring theses have been derived. Although such studies are very important, one still has to bear in mind that the transferability to infants is not unproblematic. Clinical trials in RCT quality are needed to make unambiguous statements. The prerequisites in this respect are very good in the meantime.
With every supplementation there is always the question of safety. Does this also apply for the use of HMOs in infant formula?
Comparative studies carried out by Puccio et al. with breastfed infants, who received, amongst other things, standard nutrition, as well as by Marriage et al., have shown that there is no difference with regard to important developmental parameters such as height, weight and head circumference. The novel aspect of these studies is that the HMOs used were identical to those in breast milk. Both previously used HMOs were assessed as being safe by the EFSA in Europe, as well as the FDA in the USA.
Prof. Kunz, where do you expect the greatest progress in HMO research to be made in the future? Essential clinical studies will be published in the next few months, which will either confirm or not the previously held hypotheses that were based upon in vitro investigations. In any case, the possibility to use HMOs in infant formula will become more specific. In view of the complexity of this subject matter, we should in my opinion concentrate on just a few particularly relevant HMOs.
Initial trials with infant formula and HMOs
Growth, tolerability and morbidity
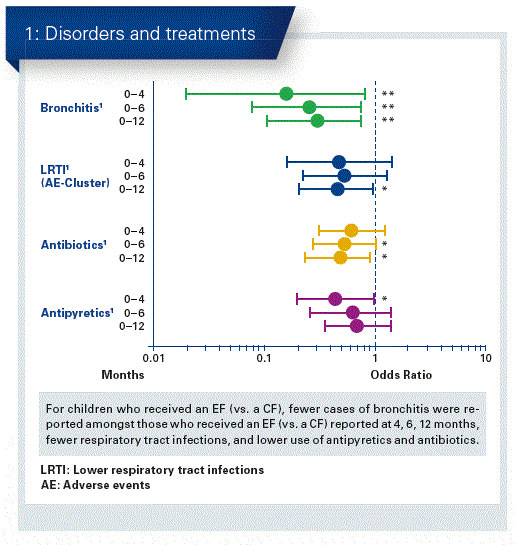
Infant formula can now be supplemented with 2’FL alone or in combination with e.g. LNnT. These HMOs are identical to those found in breast milk. They are both rated in Europe as well as in the United States as being safe (‘novel food’ status by the EFSA, GRAS status by the FDA). The aim of the study was to evaluate the safety of infant formula, which was supplemented with two HMOs, on the basis of child growth, tolerability and morbidity.Healthy newborns, 0-14 days old, received in a randomised trial either an infant formula (control group, n = 87) or the same diet, supplemented with 1.0 g/L 2’FL and 0.5 g/L LNnT (test group, n = 88) up to the 6th month. All children received a standard follow-up formula without HMOs from the 6th to the 12th month. A non-randomised group of breastfed children served as a comparison.Weight development was similar in both groups, also as were the digestive symptoms and behaviour patterns. Exceptions were seen in the test group with soft stools and more seldomly nightly awakening with 2 months. Children in the HMO group had significantly fewer cases of bronchitis reported by the parents and diagnosed by the doctor at 4, 6 and 12 months, and fewer infections of the respiratory tract during the 12 month period. The use of antipyretics over 4 months and antibiotics over 6 and 12 months was also significantly lower (Fig. 1). With regard to the presence of bacteria, a similar pattern was observed for the test and control group, although the test group was closer to the result in the breastfed group. Statistical analyses showed different occurrence rates of certain bacterial strains in the test and control groups. Clinically relevant pathogenic strains were hardly to be found, however, Clostridium difficile toxin A/B was determined almost twice as frequently in the control group than in the test group (26% vs. 14 %). Also, the metabolic ‘signatures’ between the test and breastfed group were more similar than between the test and control groups. Infant formula supplemented with 2’FL and LNnT is safe, well tolerated and promotes age-related growth. Results of a secondary outcome show a connection between HMO-supplemented nutrition and lower morbidity, especially bronchitis. Furthermore, there was a lower need for medication with antipyretics and antibiotics.
Interview
Trial on growth and tolerability
The aim of a prospective, randomised study was to clarify the effect of supplementing infant formula with HMOs on the growth of the babies and the respective tolerability. Furthermore, the caloric concentration was adjusted to that of breast milk.
Healthy single children (birth weight > 2490 g) were recorded as of the 5th day of life. The non-breastfed children received up to the 119th day randomised solely one of 3 experimental formulae. These had the same caloric, protein and fat contents, in addition to the same total amount of carbohydrates, each containing galactooligosaccharides (GOS). Two formulae were additionally supplemented with different amounts (0.2 and/or 1.0 g/L) of the HMO 2’-fucosyllactose (2’FL), the proportion of GOS was correspondingly reduced.
The three groups were compared with a breastfed reference group (n = 65). There were no significant differences in weight, height and circumference of the head. All infant formulae were well tolerated and showed average stool consistency, number of stools passed per day as well as the frequency of spitting up or vomiting after feeding. In children who received formula supplemented with 2’FL, 2’FL was present in the plasma and urine.
An infant formula with a similar calorific concentration as breast milk leads to comparable growth. Feeds supplemented with 2’FL are well- tolerated and the 2’FL profiles correspond to those of breastfed children.
Effect of 2’FL on the immune system
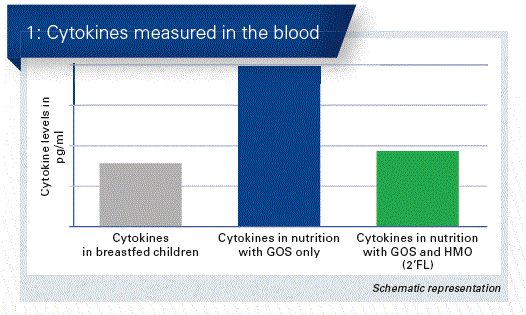
In addition, an apparent immunomodulatory effect also belongs to the many advantages of HMOs. The aim of a sub-trial to the above-mentioned RCT study was to examine on the basis of bio- markers the effect of infant formula supplemented with 2’FL on the immune system. For this sub-trial, additional blood samples were taken from the children at 6 weeks (n = 31–42/group). Cytokines are important markers for immune activity. An increased number thus indicates the presence of inflammation in the organism.
Breastfed children and those who had received a test formula supplemented with 2’FL showed no difference, although there was a significantly lower concentration (Fig. 1) of inflammatory cytokines than in the control group, which only received infant formula supplemented with GOS.
The data indicate that infant formula supplemented with 2’FL achieved lower values for inflammatory cytokines in the plasma and ex vivo, which are similar to those of breastfed children from the reference group.
Further perspectives
The effect of HMO on Rotavirus Infections
Human milk oligosaccharides have antimicrobial and immunomodulatory effects. It could be shown in piglets infected with rotavirus that HMOs can shorten the duration of rotavirus-induced diarrhoea.
In the study, the effect of HMOs and prebiotics (GOS, FOS) was measured on the immune cell population of infected and noninfected piglets. For this purpose, newborn animals received either a feed which was supplemented with 4g HMOs/L (2’-fucosyllactose, Lacto-N-neotetraose, 6’-sialyllactose, 3’-sialyllactose and free sialinic acid), or a feed with 3.6 g of short-chained galactooligosaccha- rides/L and 0.4 g long-chained fructooligosaccharides/L. After 10 days, half of the animals were infected with porcine rotavirus.
The infection changed the immune cell population. Although both feeds enriched with HMOs as well as with prebiotics show a shortening of the duration of diarrhoea in comparison to the untreated feed, there was a difference in several immune mechanisms between the groups. In particular, HMOs enhance the ileal mucosal cytokines (IFN-g, IL-10) mRNA-expression 5 days after the infection, whereas the prebiotics promoted the rotavirus-specific IhM-response. Some nutritional effects were observed in infected as well as in non-infected animals fed with HMO supplements.
Conclusion: HMOs were more effective than prebiotics in order to change systemic and gastrointestinal immune cells in pigs. These changed immune cell populations were able to influence the effects of HMOs on the susceptibility for rotavirus infections.
HMO deficiency – an indication for the risk of NEC
The incidence of necrotisising enterocolitis (NEC) is significantly lower in breastfed infants than bottle-fed premature babies. Infant formula is lacking in HMOs, such as e.g. disialyllacto-N-tetraose (DSLNT). These prevent the occurrence of NEC in animal experiments. However, it is not known up to now whether this effect also arises in human premature babies.
NEC is one of the most frequent intestinal disorders in premature babies. Reliable indications to recognise children with an increased risk early on are just as limited and urgently needed as are therapies to prevent and/or to treat NEC.
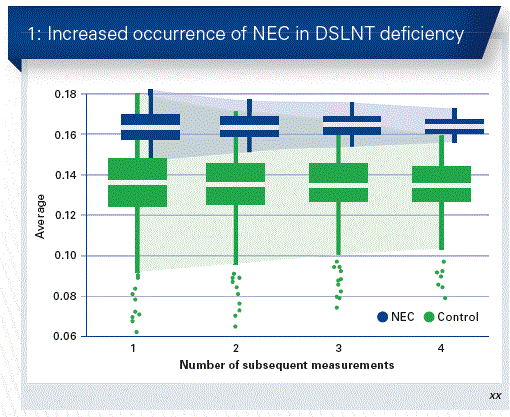
Would the hypothesis confirm that premature babies, who receive breast milk which contains lower DSLNT levels, are more likely to develop NEC than those with higher DSLNT levels? In a clinical multicenter-cohort study, 200 mothers and their premature babies with very low birth weights (VLBW) were recorded. These children were predominantly fed with mother’s milk. The HMO composition of breast milk was analysed over the first 28 days after birth, each NEC case was compared with 5 controls. 8 children of the cohort developed NEC (Bell Stage 2 and/or 3). The 4% in the study also corresponds to the statistical expectation. In these cases, the DSLNT concentrations were actually significantly lower than in the cases of the control group (Fig. 1).
Conclusion: The DSLNT content in breast milk is a potential, non-invasive indicator, in order to identify children with an increased NEC risk and to examine donor milk for its risk.
In addition, DSLNT was able to serve as a natural template for a novel therapeutics against this devastating disorder.
How do HMOs work in areas with high infection rates?
HMOs play an important role in the health of infants as substrates for advantageous intestinal bacteria. However, what is the effect of HMOs in areas where infection rates are high?
For this purpose, the maternal HMO composition and the infantile gut microbiota of 33 mother/child pairs were compared in Gambia 4, 16 and 20 weeks postpartum. The relationships between HMOs, the microbiota, infantile moridity and growth were analysed.
The data indicated that lacto-N-Fucopentao- se I is associated with a lower infantile morbidity. 3‘-sialyllactose proved to be a good indicator of the infantile weight-for- age. As HMOs, microbiota and infantile health are related, the relationship between the child’s health and the microbiome was investigated. Whereas bifidobacteria generally dominate in the gut, dialister and prevotella correlated negatively with morbidity. Bacteroids were raised in children with abnormal calprotectin levels, which is an indicator for inflammation.
It was noticeable that mothers who breastfed during the wet months of the year (July– October) produced significantly fewer HMOs than those mothers who breast-fed in the dry period (November-June).
Results:
Specific HMO types and structures
- are associated with the development of a specific microbiota
- protect against morbidity
- are growth indicators
- react to environmental conditions
HMOs promote positive bacteria and slow down detrimental pathogens
Breast milk oligosaccharides (HMOs) have a similar function in the infantile gut to prebiotics for specific species of bifidobacteria and bacteriods. However, little is known so far about the possible use by Enterobacteriaceae. These are associated with the outbreak of necrotisising enterocolitis (NEC), especially in premature babies.
In this study, the in vitro growth of pure HMOs and related carbohydrates was evaluated using individual Enterobacteriaceae strains as well as Enterobacteriaceae alliances, which were obtained from enriched piglet faeces.
None of the strains grew on 2’-Fucosyllactose (2’FL), 6_-Sialyllactose (6’SL) or Lacto-N-neo-tetraose (LNnT). However, some strains were in a position to use galactooligosaccharide, maltodextrin as well as the mono- and disaccharide components of the HMOs for growth.
Conclusion: Contrary to conventional prebiotics, 2’FL and 6‘SL did not lead to an increase in Enterobacteriaceae. They thus promote in particular selected positive bacterial strains.
Current News on Nutrition
Nutrition of the mother and infantile microbiota?
It is well known that the nutritional status of the mother, and in particular, overweight, have an influence on the growth and development of the infant.
Scientists at the Baylor College of Medicine, Houston, Texas and the University of California, San Diego, La Jolla, now assume that there is an association between changes in the breast milk microbiome and hence the HMOs which are passed on to the baby. Earlier studies had already demonstrated that nutrition which was rich in fats during pregnancy and breastfeeding has a long-term effect on the infantile gut microbiome. Does breast milk make an important contribution to this? In this study, a change in the maternal nutrition (fat vs. carbohydrates, consumption of special sugars …) was associated with changes of the breast milk microbiome and the HMOs they contain. This leads to the conclusion that the microbiota of the baby is modified by the corresponding nutrition of the mother.
After all, it is hoped in future studies that nutritional recommendations can emerge for the breastfeeding mother in order to promote a healthy microbiota for the child, according to the lead scientist for the trial.
Lack of sleep means that you pay for your sins later
A consequence of not sleeping enough at night is that you will assimilate more calories the next day.
A meta-study at King‘s College London has established a correlation between sleep deprivation and an increased caloric intake. 11 investigations with a total of 172 healthy volunteers of 18 years of age or older were included in the study, which were mostly observations made in the sleep laboratory. A part of the volunteers only slept 3.5–5.5 hours per night, compared to the control group of 7–12 hours.
For the following 24 hours, energy consumption and metabolism were determined at rest. No demonstrable effect was shown. However, the energy intake increased in the volunteers who had less sleep by an average of 385 calories (about 4 to 5 slices of bread), and they ate relatively more fat and less protein than the control group. The amount of carbohydrates, on the other hand, was similar.
Different causes for this are imaginable. A disturbance of the internal clock could lead to a release of certain hormones, which are influenced by the satiating hormone leptin and the appetite stimulating ghrelin.
If you liked this post you may also like