ESPGHAN Proceeding: Optimizing the approach to Cow’s Milk Protein Allergy
Nestlé Nutrition Institute Symposium held at the 2018 European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) meeting, Geneva, Switzerland
Optimising the Approach to Cow’s Milk Protein Allergy
State-of-the-Art in Food Allergy with Special Focus on Cow’s Milk Protein Allergy
- Professor Kirsi Järvinen-Seppo
- Division of Pediatric Allergy and Immunology and Center for Food Allergy, University of Rochester Medical Center, Rochester, NY, USA
Food allergy is an immune system-mediated adverse reaction, to food, which can be IgE-mediated, non-IgE-mediated or of mixed aetiology.
The IgE-mediated presentation is characterised by anaphylaxis, fooddependent exercise-induced anaphylaxis, urticaria, angioedema, immediate GI symptoms, bronchospasm and pollen food allergy syndrome. The non-IgEmediated response is characterised by Coeliac disease, allergic proctocolitis and food protein-induced enterocolitis (FPIES) all of which are commonly caused by cow’s milk. The mixed aetiology reactions typically present with eosinophilic oesophagitis or eosinophilic gastroenteritis, atopic dermatitis and asthma, commonly associated with consumption of cow’s milk.
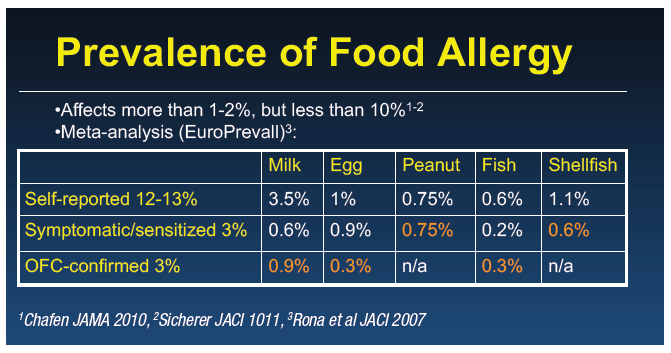
Around 6 to 8% of children are affected by food allergy, with cow’s milk, egg, and peanut being the most common. The prevalence of cow’s milk protein allergy (CMPA) varies from 1 to 10% depending on the country or region,1,2 with self-reported allergy being up to four times as common as food-challenge confirmed allergy. The disparity between self-reported and confirmed food allergy is particularly noticeable for CMPA.3
Children typically outgrow allergies to cow’s milk, egg, soy and wheat, whereas allergy to peanuts, tree nuts, fish and shellfish typically persists until adulthood, with a prevalence of about 3 to 4%.
The most important milk allergens are caseins and whey proteins, including ß-lactoglobulin and -lactalbumin. Most people with milk allergy are multisensitised, and express IgE antibodies to a number of proteins. There is a high level of cross-reactivity between the proteins of cow, goat and sheep milk, but less so between cow, camel, and pig milk and even less between cow, horse and donkey milk.4
IgE-mediated food allergy is the most common, and its diagnosis is straightforward as food-specific IgE testing or a skin prick test is typically positive for immediate reactions. The best treatment is avoidance and selfadministered epinephrine for emergencies.
Diagnosis in non-IgE-mediated and mixed aetiology food allergies is not based on specific IgE. In allergic proctocolitis, blood streaked mucousy, loose stools appear up to six months of age in breast-fed or formula-fed infants who are otherwise well.5-7 The inflammation is eosinophil-dominant and cow’s milk and soy are the most common triggers, although viruses can produce a similar reaction. Bleeding resolves within three days following complete elimination of the allergen from the infant’s diet, and sometimes that of the mother.
Acute FPIES typically starts in early childhood and presents with emesis 2 to 4 hours after ingestion of the food protein, usually cow’s milk, soy, rice or oats. Diarrhoea may follow within 8 to 24 hours and there is often an appearance of sepsis, with lethargy, limpness, and the risk of shock due to dehydration. There is an increased white cell count, acidosis and methemoglobulinaemia in around 15% of cases.
In chronic FPIES, which occurs earlier at around 1 to 3 months, there is watery diarrhoea, failure to thrive, mucousy stools with intermittent emesis associated with continuous exposure to cow’s milk, or soy formula. Blood tests often reveal a low total protein.
Allergic eosinophilic gastroenteropathy affects either the oesophagus or the lower GI tract. Allergic eosinophilic oesophagitis can present from infancy to adulthood with reflux symptoms, dysphagia, failure to thrive, irritability, and vomiting. Allergic eosinophilic gastroenteritis generally appears up to adolescence and is characterised by vomiting, pain, anorexia, hematemesis, and even hypoalbuminaemia. The most common trigger for both is cow’s milk.
At a molecular level, IgE-mediated allergy begins with digestion of the protein, followed by absorption and processing by antigen presenting cells before presentation to T cells. The T cells, particularly Th2 type cells orchestrate the B cells to produce specific IgE antibodies to milk protein which are then bound by high affinity IgE receptors on mast cells. Upon re-exposure to the same foods, parts of these allergens (epitopes) cross link consecutive IgE molecules, resulting in the release of lipid mediators such as histamine and tryptase, which are responsible for the typical manifestations of flushing, vasodilation, pruritus, bronchoconstriction and vascular permeability.
T cells also synthesise lipid mediators such as IL-4 which is responsible for mast cell up-regulation. IgA and IgG antibodies can suppress this reactivity by blocking antibodies and neutralising food antigens, and by T regulatory cells which suppress Th2 and mast cell activity. These molecules are key to developing tolerance to food allergens.
The non-IgE-mediated presentation has much more variable aetiology and is not so well understood.
Food allergies are a growing clinical problem, with a doubling of peanut cases in the US from 1997 to 2003, and a further doubling to 2008,8,9 and an 18% increase in all food allergies.10 There is similar data for Canada, United Kingdom, and Australia.11-13 The main increase is seen for peanut and tree nuts (three to four-fold), compared to a two-fold increase for milk and egg allergy.
A 2016 paper proposed three different hypotheses to account for the increase in food allergy.14 One hypothesis suggests that reduced levels of vitamin D may be responsible for the development of more allergic diseases and asthma. The hygiene hypothesis proposes that exposure to a less diverse microbiological environment might induce intolerance to allergens.
The dual allergen exposure hypothesis proposes that competing routes of exposure have opposite effects on the development of sensitisation and tolerance. Normal development of CD103+ dendritic cells induces tolerance in the GI tract, therefore most people become tolerant to ingested food through the development of T regulatory cells. However, skin allergen exposure can also contribute to sensitisation especially in those with filaggrin mutation, or significant eczema. Through daily exposure to food proteins, dendritic cells in people with abnormal skin barrier function, can up-regulate IL-4 cytokines in the Th2 type memory. Although a person may rigorously avoid exposure via the oral route there can be continuing allergen exposure through the skin, which can be particularly sensitising when oral exposure is limited.
Introduction of peanuts to the diet of high risk infants with significant eczema can be a powerful tool for inducing tolerance.15 However, it is not known whether the same applies to other food allergens. Most children who develop CMPA also have eczema: if there is early introduction to the allergen in the first weeks of life this may help to develop tolerance.
There may be a window of opportunity that includes not just infant feeding practices, decreasing C-sections and early life antibiotics, but also maternal exposure, environment, pets, lifestyle, diet, allergen and microbial exposures that can shape the immune system to become tolerant or sensitised.16 There is good data to suggest that farm life can protect from the development of asthma and allergic rhinitis. It is not clear whether this also protects from food allergy, although our work is continuing on this topic.
ß-lactoglobulin is found in very small concentrations in some women’s breast milk, and is related to maternal diet.17-20 ß-lactoglobulin induces symptoms such as eczema and rashes in some highly sensitised babies.20-22 When cow’s milk is eliminated from the mother’s diet, the eczema clears, while re-exposure to milk protein through the maternal diet causes the rashes to re-appear.
It is possible that low levels of antigen in breast milk could bring about sensitisation, but if they are ingested along with tolerance-inducing breast milk immune factors such as IgA, TGFß and HMOs, the effect could be to develop tolerance.21,22 This subject is still under investigation.
The prognosis for CMPA is generally favourable. Eighty per cent of children outgrow their IgE-mediated CMPA by early adolescence and recurrence is extremely uncommon.23 Development of tolerance is probably due to recognition of linear epitopes on milk proteins:24 tolerance of baked milk is a good prognostic sign.25 Non-IgE-mediated manifestations such as proctocolitis are usually outgrown by 1 year, FPIES by 3 to 4 years. However, the prognosis for eosinophilic oesophagitis is more variable and poorly understood.
The Challenge of Correct Diagnosis and Management of Cow’s Milk Protein Allergy
- Professor Sibylle Koletzko
- Dr von Hauner Children’s Hospital, Ludwig-Maximilian University, Munich, Germany
It is very important to arrive at a correct diagnosis for cow’s milk protein allergy (CMPA), to ensure appropriate treatment and monitoring, however, symptoms can be very broad and non-specific. In 2012 ESPGHAN published practical diagnostic and management guidelines for CMPA.26
We carried out a survey to assess awareness of the guidelines, whether they were followed, and whether they were useful. Paediatricians in 13 European countries were invited via their medical associations to answer some basic demographic questions, and to respond to a number of questions on medical cases.
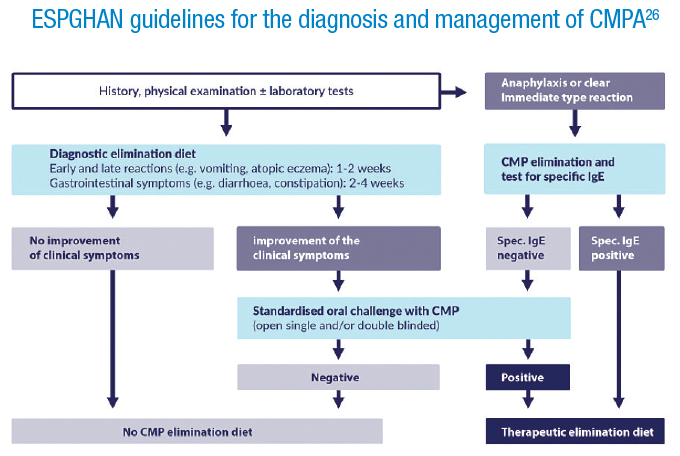
Over 2500 completed assessments were returned. There were more females (72%), and the largest age group was 55 to 64 years, both of which are representative of the respective societies. The majority, 72%, worked in paediatric practice, and had more than 20 years’ experience. There were very few sub-specialists, only 4% were allergy specialists, so the majority of responders would be the first point of contact for children suspected of having a food allergy.
“In which circumstances would you switch a 3-month old formula-fed infant with suspected CMPA from a normal infant formula to a special therapeutic formula?”
A number of possible responses were given and multiple answers were allowed.
Of the given options, failure to thrive (poor feeding, weight loss of 300g over the previous three weeks) was most appropriate to the guidelines and was selected by 53% of respondents. Forty-three percent also voted for moderate atopic eczema on the face and trunk. However, the most popular choice, 60%, was blood-streaked stools for one week.
Rectal bleeding is an alarming symptom. However it is seen in breast-fed and non-breast-fed infants without any other signs of organic disease.27 Studies have found that in the majority of young infants, rectal bleeding is of short duration without any intervention.27,28 When children with short-term rectal bleeding were examined it was found that the majority did not have eosinophilic colitis and had normal histology and macroscopic findings.27,28 About 20% were subsequently found to have CMPA.
Excessive crying was chosen by 25% but a recent systematic review of crying patterns in infants found that two hours is quite normal for a baby of three months.29 If the crying were to persist beyond four months of age or is accompanied by other symptoms, it would be sensible to investigate further.
“A 10 month old infant has a history of chronic diarrhoea and failure to thrive. Which investigation is most useful to rule out a diagnosis of CMPA?”
Only one answer was allowed and most respondents, 68%, chose the correct answer which is to eliminate dairy products from the diet and to carry out a challenge test if the infant appears to improve. A negative blood test for IgE specific for cow’s milk protein does not rule out CMPA, and it is not possible to exclude or prove CMPA using skin prick tests: it must be followed by an elimination and re-challenge procedure. Endoscopy with small bowel biopsies and elimination of lactose from the diet were only selected by a few respondents and are not recommended.
“The same child is diagnosed with CMPA but refuses to take the extensively hydrolysed formula. Which alternatives may be given to the child?”
The three correct options are soy-based formulas (chosen by 51%), aminoacid based formula (62.5%) and milk-free rice-based baby cereals (56.5%). It is of concern that 11% selected goat’s milk formula, as there is a high degree of cross reaction to cow’s and goat’s milk: 90% of children with CMPA also react to goats milk, which is therefore a dangerous option. A partially hydrolysed infant formula is also not safe in children with CMPA since many have an allergic reaction to the whey protein and peptides in these formulas.30
“A ten-week old exclusively breast-fed child with frequent regurgitation (4 to 5 times per day) is reported to have pain and to be uncomfortable. Clinical examination is normal, while length is within the 75th percentile and weight within the 50th percentile. How would you proceed?”
Three-quarters of respondents chose the recommended option which was to see the child again in two weeks for re-examination. Frequent regurgitation without any alarm symptoms in a ten week old is not a sufficiently i reason to switch to a hydrolysed or amino acid formula (selected by 8%). A trial of acid suppressive therapy has been found to be no better than placebo in this population.31
“A five-month old breast-fed infant develops swelling of the lips and eyelids after drinking his second bottle of infant formula. Which alternative milk or infant formula would you recommend?”
The recommendation is to go back to complete breast-feeding (selected by only 26% of respondents). The most common choice (46%) was to recommend breast-feeding, but also to advise the mother to eliminate dairy from her own diet. As there is no risk of anaphylaxis through ingestion of the mother’s milk, we recommend the mother remain on a normal diet. If the baby subsequently has a skin reaction then she should consider reducing or eliminating milk from her own diet. But small amounts of dairy in the maternal diet may induce tolerance in the infant and therefore may be beneficial in the long run.
Other options were an extensively hydrolysed formula, an amino-acid based or a soy-based formula. These are acceptable choices if the mother cannot resume breast-feeding.
“The mother in the previous case wishes to start weaning the child. What would you advise?”
Fifteen and 25% of respondents respectively would avoid other potential allergens such as eggs or fish, or would not introduce complementary feeding before six months. But recent guidelines suggest that there is no reason to delay the introduction of solid foods in children with CMPA. The recommended approach is to introduce complementary feeding in the same way as with non-allergic infants, but to avoid any products with cow’s milk protein (chosen by 53%).
“The child in the case above undergoes a blood test which is negative for specific IgE. Can the mother re-introduce dairy products to the child’s diet?”
A negative test for cow’s milk specific IgE does not always indicate an absence of allergy, so dairy products should not be re-introduced on this basis (selected by 7%). Thirty-six percent of respondents chose to recommend elimination of dairy products for the first year on the basis of the acute reaction experienced by the child. However, the recommended advice (chosen by 46%) is first to confirm the diagnosis with elimination of dairy products and then to rechallenge in children with a negative test for specific IgE. If the challenge is positive a therapeutic elimination diet until one year of age is justified.
In a child with a clear acute reaction, and a positive skin prick test, there is no need for a challenge before proceeding with therapeutic elimination. But if the test is negative then confirmation is needed to rule out other alternatives, such as a reaction to cereal, porridge or other products.
To summarise: CMPA is common, affecting 2 to 3% of children, but diagnosis can be difficult. Symptoms are non-specific, such as eczema (50% of cases), gut dysmotility (diarrhoea and constipation), frequent regurgitation, abdominal pain, refusal to feed, malabsorption and failure to thrive. Diagnostic proof is essential: improvement must be seen upon elimination of cow’s milk products, followed by an adverse response to a cow’s milk protein re-challenge after symptoms have subsided. Endoscopy and histology can be helpful but are rarely needed. A positive specific IgE or skin prick test is indicative but does not provide a diagnosis; while a negative test for specific IgE does not exclude the diagnosis. Our survey results demonstrated that not all paediatricians are aware of these points and further education is needed.
The Gut Microbiome in Infants with Cow’s Milk Protein Allergy – Implications for Treatment
- Doctor Christina West
- Department of Clinical Sciences, Pediatrics, Umeå University, Sweden
The greatest microbial influence to which the neonate is exposed is the maternal microbiota during delivery, although there is emerging evidence that the process of exposure may begin in the womb, imprinting the microbiota of the offspring and the immune system in preparation for birth.32 During infancy the baby continues to be exposed to the maternal microbiota through breast milk. The microbial colonisation of the mucosal surfaces in the baby’s gastrointestinal tract will have a large influence on immune maturation.
A number of maternal factors influence establishment of the baby’s microbiota, including maternal antibodies and nutrients, the transference of which influence expansion of the gut innate immune cells, development of intestinal epithelial cells, mucus development and the expression of antimicrobial peptides and the secretion of antibodies.33 Together, these will prevent translocation of the endogenous microbiota and avoid hyper-reactivity to micro-organism derived compounds and ultimately, food antigens.
In a healthy breast-fed baby the development of the microbiome is quite predictable. The early gut microbiota is abundant in Bifidobacteria and the microbes that characterise the early stages of development are more capable of metabolising nutrients associated with breast-feeding, such as simple carbohydrates. In the later stages the infant will have a gut microbiota that can digest solid foods.34
In the early years there is a successive establishment of the gut microbiota. The facultative anaerobes such as E coli and Streptococcus species that dominate in the first few days, are followed by Bifidobacteria, Bacteroides and Clostridium species due to oxygen deprivation.35 This is followed by successive establishment of new bacterial taxa and increased diversity which progresses into the school years.
Several maternal factors influence susceptibility to allergic disease in infancy, including the allergy phenotype, the maternal microbiota, mode of delivery of the infant, and breast-feeding. There is increasing evidence that dysbiosis of the intestinal microbiota in infancy, and reduced diversity precedes the clinical manifestations of sensitisation, eczema, food and respiratory allergies. However, a causal relationship has not been established.35
The establishment of the microbiota parallels the development of innate and adaptive immune pathways. In individuals with high biodiversity there is an increase in short-chain fatty acid (SCFA) production that has both nutritive and inflammatory effects and brings about induction of the T regulatory cells. In individuals with low biodiversity and dysbiosis, IgE production and proinflammatory responses are enhanced.36
The CHILD (Canadian Healthy Infant Longitudinal Development) study compared the gut microbiome of 166 children through faecal sampling at three and 12 months and looked at how this might be related to sensitisation to food substances.37 Skin prick testing at one year identified twelve infants who were sensitised to at least one common food allergen. In the gut microbiota of food-sensitised infants, Enterobacteriaceae were over-represented, while Bacteroidaceae were under-represented. The authors concluded that low gut microbiota richness and an elevated Enterobacteriaceae / Bacteriodaceae ratio in early infancy are associated with subsequent food sensitisation.
An interesting area of current study is that of the toll-like receptors (TLRs) that we believe are the ancient “gate keepers” of innate immunity. They are expressed on epithelial cells, endothelial cells and on leukocyte subsets in the blood. TLRs have evolved in mammals to recognise conserved structural molecules unique to microbes, allowing them to detect the presence of infection and to induce activation of inflammatory and antimicrobial innate immune responses. Recently it has been shown that T regulatory cells also express TLRs. When these TLRs are activated they can increase or decrease the suppressor activity of T regulatory cells providing an important link between innate and adaptive immunity.
It has been reported that allergic children show exaggerated inflammatory responses to TLR-activation before the onset of allergic disease, but these exaggerated responses decrease with age. The early hyper-responsiveness fails to translate into a corresponding maturation of TH1 function which remains attenuated compared to that seen in non-allergic subjects.38 It has also been demonstrated that allergic children have immature T regulatory functions.39
We hypothesised that this deviant innate immune maturation might be dependent on microbial exposure. In a nested case control study in a high risk cohort in Australia, stool samples were taken from atopic mothers in the third trimester of pregnancy and their infants were followed for the first two and a half a years of life.40 Stool samples were taken from the infants at one week, and at one and twelve months and the infants were clinically assessed at repeated intervals until two and half years of age. The development of the microbiome in children that were sensitised to milk, egg, or peanuts and also had eczema, was compared to that of healthy controls, who had no sensitisation, eczema, or other allergic manifestations.
Blood samples were taken at six months and were cultured with specific microbial ligands for TLR2, a principle receptor for Gram positive bacteria, and TLR4 which is the principle receptor Gram negative bacteria. With infants who developed IgE associated eczema and food sensitisation, it was found that the mothers also had a lower -diversity of the main bacterial group, Bacteroidetes, in their stool. There was also a reduced abundance of Enterobacteriaceae in the infants who developed atopic eczema, and an inverse association between levels of Enterobacteriaceae TLR-4 induced TNF-. This suggests that when there is a reduced abundance of potentially immunomodulatory bacteria in the gut this may be associated with exaggerated inflammatory cytokine responses to TLR ligands and may influence the development of innate immune response patterns.
There are also some interesting associations between the gut microbiome and cow’s milk protein allergy (CMPA). An observational study carried out by the Consortium of Food Allergy study involved 226 children who were enrolled in infancy. All of the involved children had IgE-mediated CMPA, and the children were followed with clinical evaluation, milk-specific IgE levels, skin prick test and stool samples at entry, 6 months, 12 months and then annually until eight years of age. Milk allergy had resolved at this age in 56.6% of the children.41
Using principal coordinate analysis (PCoA) there was a distinct gut microbiome in three to six month old subjects whose allergy had resolved, compared to those whose allergy persisted. This was not seen in subjects sampled later on at seven to 12 months. Taxa within the Clostridia class in the Firmicutes phylum were enriched in children with resolution of milk allergy, while Bacteroidetes and Enterobacter were seen in subjects whose milk allergy did not resolve. This demonstrates that early microbiome composition is associated with the development of tolerance versus persistence of milk allergy. However, the resolution of this analysis was low. In a more recent study there have been efforts to attempt a higher resolution. In a study of 82 children with atopic dermatitis, with and without food allergy, faecal samples were analysed using 16S rRNA microbial analysis.42 Four microbial species were found to be under-represented in children with confirmed food allergy: Bifidobacterium breve, Bifidobacterium adolescentis, Faecalibacterium prausnitzii, and Akkermansia muciniphila. Notably, in one of our recent studies, Faecalibacterium prausnitzii was associated with a tolerogenic systemic immune response.43
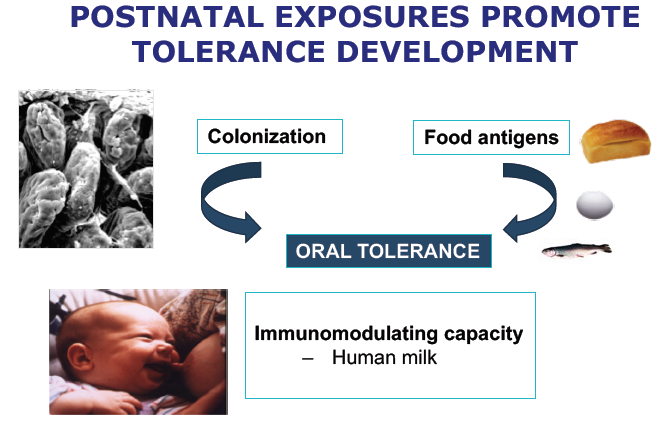
It is clear that post-natal exposure will promote development of oral tolerance through colonisation of the gut and the introduction of food antigens. Human milk has a plentiful supply of bioactive factors with immunomodulating capacity.
The aim of intervening to treat food allergy and CMPA is to induce a tolerogenic environment in the gut. This can be achieved by dietary interventions such as the addition of fibre, pre-, pro-, and synbiotics or lactose to infant formula. Human milk is plentiful in lactose and complex carbohydrates which have many bioactive properties, so the addition of lactose and human milk oligosaccharides (HMOs) can also be beneficial. The interventions should increase mucus production, reinforce gut barrier integrity, stimulate the production of SCFAs, stimulate or dampen the innate immune responses, induce T regulatory cells and dampen reactive clones.40,44
To summarise, variations in the gut microbiota have been implicated in the development of CMPA and microbial signatures have been associated with tolerance acquisition. However at this stage meta-genomic studies are needed to enable higher resolution of the microbes involved and for the understanding of functional relevance. Prebiotics (including lactose), probiotics and synbiotics may influence gut microbiota composition in cow’s milk allergy and HMOs may have therapeutic potential in the treatment of CMPA.