Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity
Key Messages
- Human milk oligosaccharides (HMO) are a predominant component of human milk and are comprised of diverse structures that are neutral or acidic and some forms are sialylated or fucosylated, which contributes to their biological functions.
- HMO protect the infant from pathogenic infections, facilitate the establishment of the gut microbiota, promote intestinal development, and stimulate immune maturation.
- Some types of HMO are now commercially available and are being added to infant formula alone or in combination with other prebiotics.
Keywords
Human milk oligosaccharides, Immunity, Infant
Abstract
The immune system of the infant is functionally immature and naïve. Human milk contains bioactive proteins, lipids, and carbohydrates that protect the newborn and stimulate innate and adaptive immune development. This review will focus on the role human milk oligosaccharides (HMO) play in neonatal gastrointestinal and systemic immune develop ment and function. For the past decade, intense research has been directed at defining the complexity of oligosaccharides in the milk of many species and is beginning to delineate their diverse functions. These studies have shown that human milk contains a higher concentration as well as a greater structural diversity and degree of fucosylation than the milk oligosaccharides in other species, particularly bovine milk from which many infant formulae are produced. The commercial availability of large quantities of certain HMO has furthered our understanding of the functions of specific HMO, which include protecting the infant from pathogenic infections, facilitating the establishment of the gut microbiota, promoting intestinal development, and stimulating immune maturation. Many of these actions are exerted through carbohydrate-carbohydrate interactions with pathogens or host cells. Two HMOs, 2’-fucosyllactose (2’FL) and lacto-N-neotetraose (LNnT), have recently been added to infant formula. Although this is a first step in narrowing the compositional gap between human milk and infant formula, it is unclear whether 1 or 2 HMO will recapitulate the complexity of actions exerted by the complex mixture of HMO ingested by breastfed infants. Thus, as more HMO become commercially available, either isolated from bovine milk or chemically or microbially synthesized, it is anticipated that more oligosaccharides will be added to infant formula either alone or in combination with other prebiotics.
Background
The human infant enters the world with a functionally naïve immune system affecting both adaptive and innate immune responses [1] , which leaves the newborn at high risk for common infections. Postnatal immune maturation is stimulated by antigenic exposures and host-microbe interactions [1, 2]. How and what the infant is fed influences the development and competence of the immune system [3–5]. Human milk protects the infant during this vulnerable period by providing bioactive components that protect the infant from pathogenic infection, support intestinal development, promote barrier function, stimulate immune development, facilitate immune tolerance, and feed gut microbes [2–5]. Thus, human milk supplies multiple layers of protection for the infant ( Fig. 1 ).
Breastfeeding, particularly exclusive breastfeeding for 6 months or more, relative to formula-feeding, decreases the incidence and/or severity of infectious diseases [6]. Many diseases with infectious and immune components in their etiology, including diarrhea, respiratory and urinary tract infections, otitis media, bacteremia, and necrotizing enterocolitis occur less often in breast than formula-fed infants [6, 7]. Breastfeeding has also been implicated in reducing the incidence of other diseases involving the immune system and immune tolerance, such as inflammatory bowel disease, celiac disease, asthma, allergy, type 1 diabetes, as well as acute lymphoblastic and acute myeloblastic leukemias [6, 8]. These benefits may be mediated in part through effects of breastfeeding on the intestinal microbiota [8, 9] , which in turn stimulates maturation and specificity of the neonatal mucosal and systemic immune systems [2].
The immune benefit of breastfeeding has been attributed in part to the diverse bioactive components in human milk [2–5]. A strong case can be made for a key role of human milk oligosaccharides (HMO) in neonatal immune defense and maturation. As will be described below, HMO are present in high concentrations in human milk, exist in an incredible structural diversity [10–13], and confer host protection and mediate immune responses through a number of mechanisms [14, 15].
HMO Content and Composition
HMO are complex soluble glycans that are predominantly present in free form in milk. These glycans are synthesized from 5 basic monosaccharides: galactose, glucose, N-acetylglucosamine, fucose, and the sialic acid derivative N-acetylneuraminic acid [10, 11]. With few exceptions, all HMO carry lactose (Galβ1–4Glc) at the reducing end, which can be elongated in β1–3 or β1–6 linkage by 2 different disaccharides, either Galβ1–3GlcNAc (type 1 chain) or Galβ1–4GlcNAc (type 2 chain) [11].
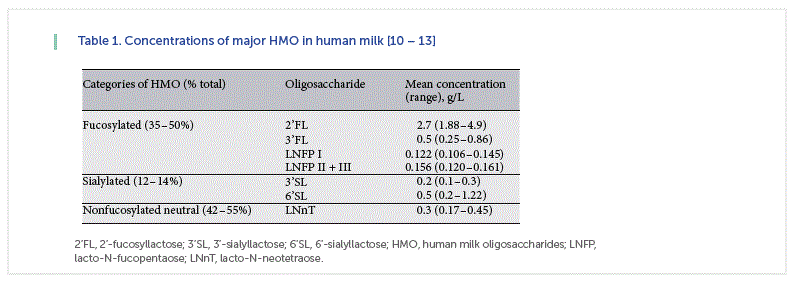
The HMO content has been reported in the range of 1–10 g/L in mature milk and 15–23 g/L in colostrum [10–13]. In term breast milk, ~35–50% of HMO are fucosylated, 12–14% are sialylated, and 42–55% are nonfucosylated neutral HMO [10–13] ( Table 1 ). However, HMO composition is influenced by maternal genetics, including secretor and Lewis Blood Group status [10, 11]. HMO fucosylation is mediated by the 2 fucosyltransferases FUT2 (secretor gene) and FUT3 (Lewis gene). Nonsecretor mothers, who lack a functional FUT2 enzyme and represent about 30% of women worldwide, produce milk lacking in α1-2-fucosylated oligosaccharides like 2α -fucosyllactose (2’ FL) and lacto-N-fucopentaose (LNFP) I [10, 11]. The absence of these compounds may have functional consequences. For example, infants consuming milk produced by women who are nonsecretors exhibit delayed colonization of bifidobacteria, higher abundance of Streptococcus taxa, and have functional differences in the metabolic activity of their microbiota [16]. Infants fed milk from nonsecretor mothers are at higher risk for diarrheal diseases [17].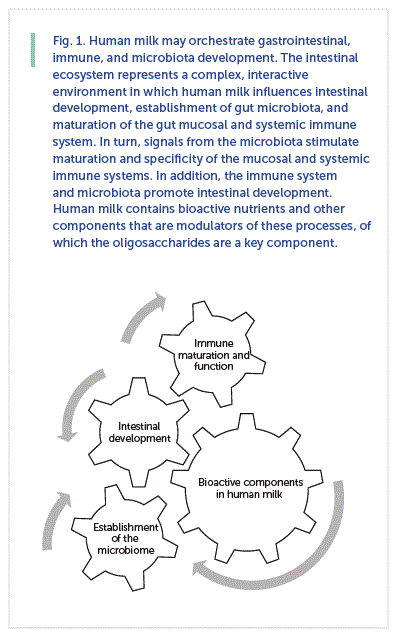
HMO and the Microbiome
The development of the infant gut microbiota is a sequential process that begins in utero and continues during the first 2–3 years of life. Microbial composition and diversity is shaped by host genetics and multiple environmental factors, of which diet is a principal contributor [8, 9]. Studies conducted over the past decade have shown that specific Bacteroides and Bifidobacterium species that commonly colonize breastfed infants efficiently utilize HMO as carbon sources. This is particularly true of B. longum ssp. infantis (B. infantis), which is a predominant gut microbe in most breastfed infants [18]. The discovery of a genomic island in B. infantis that encodes specific enzymes for the metabolism of HMO supports an adaptation of this species to the intestinal milieu of the breastfed infant [18, 19]. Indeed, a recent study in human infants fed formula supplemented with 2’ FL (1 g/L) and LNnT (0.5 g/L) demonstrated that the global microbiota composition of infants fed formulae with 2’ FL and LNnT was significantly different to that of infants fed nonsupplemented formula (p < 0.001) at the genus level and closer to that of breastfed infants at 3 months of age [20]. In addition, Bifidobacterium was more abundant (p < 0.01), whereas Escherichia and unclassified Peptostreptococcaceae were less abundant in infants fed formula with 2’ FL and LNnT compared to infants fed nonsupplemented formula, and these levels were closer to those observed in breastfed infants [20]. Furthermore, the concentrations of several important metabolites in stool (propionate, butyrate, and lactate) in infants fed the HMO-supplemented formula were more similar to those of breastfed infants [20].
Previously, we have shown that HMO fermentation by neonatal pig microbiota produced short-chain fatty acids and promoted the growth of beneficial bacteria in vitro [21] and in vivo [22]. Gut bacteria and the immune response, particularly the gastrointestinal immune response, are tightly interrelated [23]. Thus, in this animal model, HMO-induced changes in the gut bacterial populations of the pigs could alter the course of an intestinal infection [24] which in turn would alter the immune response [22]. Alternatively, the change in the gut bacteria could directly affect the immune system of these animals [2]. Additional ways whereby HMO may mediate neonatal immunity are summarized in the following section.
HMO as Immune Modulators
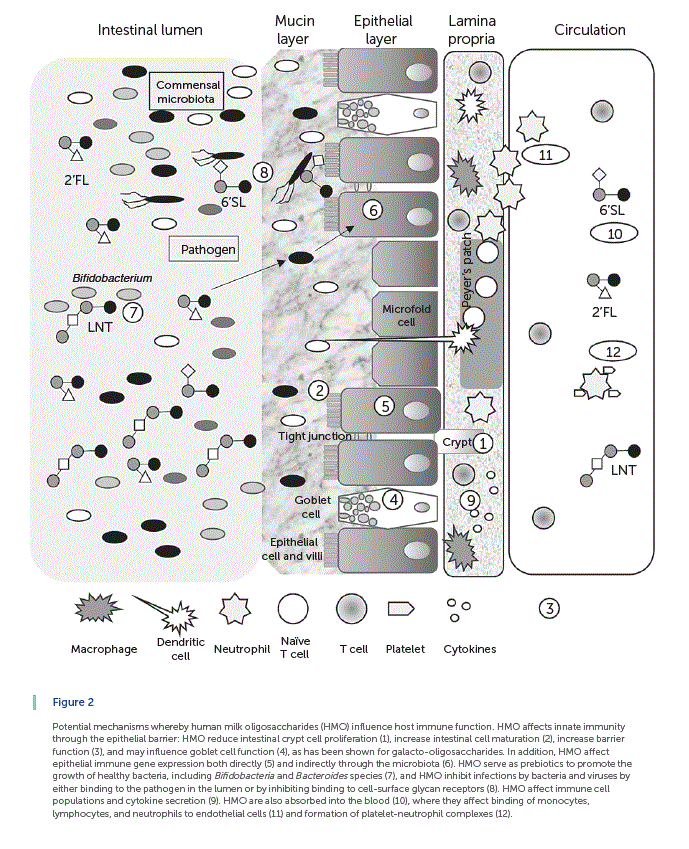
Summarized in Figure 2 are the results of an accumulating body of evidence showing that HMO indirectly and directly influence infant mucosal and systemic immune function. In general, intestinal health and barrier function are considered a first line of defense in innate immunity. Cell proliferation takes place in the crypts, and cells differentiate as they migrate up the villus-crypt axis, with the exception of Paneth cells, which migrate down to the base of the crypt. HMO reduce intestinal crypt cell proliferation [25, 26] , increase intestinal cell maturation [26] , and increase barrier function [26] (indicated by 1–3 in Fig. 2 ). A layer formed by mucus glycoproteins or mucins produced by goblet cells acts as a lubricant and a protective physical barrier between the intestinal epithelium and the luminal contents (indicated by 4 in Fig. 2 ). HMO may influence goblet cell function, as has been shown for galacto-oligosaccharides (GOS) [27]. HMO affects epithelial immune gene expression both directly [28–30] and indirectly through the microbiota [31] (indicated by 5 and 6 in Fig. 2 , respectively). As noted above, HMO serve as prebiotics to promote the growth of healthy bacteria, including Bifidobacteria and Bacteroides genera [32] (indicated by 7 in Fig. 2 ), and HMO inhibit infections by bacteria and viruses by either binding to the pathogen in the lumen or by inhibiting binding to cell-surface glycanreceptors [14–15, 22] (indicated by 8 in Fig. 2 ). Additionally, dietary oligosaccharides decorate the intestinal lining contributing to the intestinal glycan repertoire [33]. HMO also contribute to epithelial barrier function by supporting the growth of B. infantis in the infant gut [10, 18]. B. infantis produces peptides that have been shown to normalize intestinal permeability through enhanced tight junction protein expression in a mouse model of colitis [34]. It is likely that HMO support other bacterial species that are important for the maintenance of gut integrity. These changes in intestinal barrier function would, in turn, alter both the local and systemic immune system [35]. HMO affect immune cell populations and cytokine secretion [22, 36] (indicated by 9 in Fig. 2 ). Some HMO are also absorbed into the blood stream [37–39] (indicated by 10 in Fig. 2 ), where they exert systemic effects by binding of monocytes, lymphocytes, and neutrophils to endothelial cells [40] (indicated by 11 in Fig. 2) and formation of platelet-neutrophil complexes [41] (indicated by 12 in Fig. 2 ). Readers are referred to a recent review by Kulinich and Liu [15] for additional discussion of this topic.
Carbohydrate Binding as a Potential Mechanism of HMO in the Immune System
Carbohydrates and carbohydrate-binding proteins play an important role in immune responses. Cells have unique glycan signatures made from combinations of specific glycan motifs that are engaged when a cell contacts another cell or other components of its environment [42, 43]. However, many of the glycan motifs found on mammalian cells are also found on microbes and in food, including human milk. These similarities provide opportunities for host-microbe-HMO interactions.
Lectins are carbohydrate-binding proteins on the surfaces of mammalian cells that translate recognition of specific motifs and the spatial presentation of those motifs into action. Lectins are grouped according to their carbohydrate recognition domains (CRD) [42, 43].
There are at least a dozen CRD identified in mammals, but 3 classes of lectins related to the influence of HMO on immune responses are C-type lectins, siglecs (sialic acid-binding Ig-like lectins), and galectins.
C-type lectins require calcium to function and include selectins, mannose-binding lectin, and dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN). C-type lectin receptors on the surface of dendritic cells (DC) determine whether the cell will induce tolerance rather than lymphocyte activation [44]. DC-SIGN is of particular interest with regard to mechanisms by which HMO can influence immunity because it has a CRD specific for fucose units. Furthermore, DC-SIGN is expressed by cells in the gastrointestinal tracts of infants [45]. These intestinal cells are likely antigen-presenting cells as DC-SIGN is expressed by antigen-presenting cells, specifically DC [43]. Although interactions between fucosylated ligands and DC-SIGN contribute to immune tolerance, the cellular response ultimately depends upon the other ligand-receptor reactions occurring simultaneously [43]
Siglecs are sialic acid-binding lectins most commonly found on subsets of immune cells [46]. There are at least 16 siglecs expressed by different leukocyte populations, which include sialoadhesin (siglec-1), CD22 (siglec-2), myelin-associated glycoprotein (MAG, siglec-4), siglec-15, and CD33-related siglecs. Siglec specificity derives from differences in secondary binding sites [43]. Siglecs are endocytic cell surface receptors that carry cargo between the cell surface and intracellular vesicles; these receptors are mainly expressed on cells involved in antigen processing and presentation [43]. Furthermore, sialic acid-containing molecules can gain entry to macrophages by binding to siglecs on the cell surface [46]. On mammalian cells, some sialic acid-containing glycans function as self-associated molecular patterns and prevent immune responses to nonpathogenic stimuli. Ligation of particular siglecs stimulates the production of the immunoregulatory cytokine interleukin (IL)-10 [47].
Galectins are important for cell turnover and immune regulation. The CRD of galectins is specific for β-galactosides. When cells are desialylated, the density of exposed galactose moieties on the cell surface increases. For example, naïve T cells express CD45 with an α-2,6-linked sialic acid. The amount of α-2,6-linked sialic acid is reduced following T-cell activation. The decrease in α-2,6-linked sialic acid renders the activated T cells susceptible to galectin-1- mediated apoptosis [48]. Thus, binding of sialyated HMO to cells may prevent apoptosis.
HMO as Modulators of Mucosal Immunity
Intestinal cell lines have been used to determine effects of HMO on immune-related gene expression and protein production. These cells have been co-incubated with oligosaccharides [28] , bacteria [48] , or lipopolysaccharides (LPS) to model a bacterial infection [29]. Co-incubation of Bifidobacterium with cells of the Caco-2 intestinal cell line and HMO resulted in downregulation of intestinal cell genes related to chemokine activity compared to co-incubation with glucose or lactose [29]. Conversely, in the absence of a bacterial co-stimulant, HMO increased expression of several chemokines by the HT-29 cell line [28]. Additional work in T84 and HCT8 intestinal cell lines showed that complex mixtures of HMO as well as 2’ FL reduced signatures of intestinal inflammation [29].
HMO have been demonstrated to affect the course of a gastrointestinal viral infection. In an acute rotavirus (RV) infection model where a 21-day-old piglet’s ileum was isolated in situ, intestinal loops co-treated with HMO and RV had reduced non-structural protein-4 (NSP-4) mRNA ex-pression indicating that HMO can reduce RV replication [49]. Intestinal cytokine and chemokine expression, however, was not affected. Both neutral and acidic HMO decreased NSP4 intestinal mRNA expression in the in situ model, whereas only acidic HMO effectively inhibited RV infectivity in an in vitro model [49].
HMO as Modulators of Systemic Immunity and Protection from Infection
HMO are detected in the plasma of infants fed human milk at concentrations of 1–133 mg/L [37, 39] , suggesting the potential for dietary HMO to directly affect immune cells circulating in the blood. As discussed above, many immune receptors recognize the oligosaccharide structures of their glycoprotein ligands [14, 15]. Because a subset of HMO is structurally similar to selectin ligands [14] , it is likely that HMO can bind directly to immune cells and trigger signaling that results in changes to immune cell populations and functions. For instance, the P- and E-selectins recognize sialyl-Lewis x (sLeX), a glycan moiety of several HMO [11]. Additionally, fucosylation and sialylation, 2 enzymatic modifications common to HMO, enable binding to selectins [50]. HMO- induced disruption of immune protein-carbohydrate interactions reduced neutrophil rolling [40] and activation [41]. HMO directly affect immune cell proliferation and cytokine production in ex vivo experiments with peripheral blood mononuclear cells (PBMC) from neonatal pigs [36].
Stimulation with isolated HMO stimulated production of the regulatory cytokine IL-10 [36]. Others observed that the acidic HMO induce IL-10 production; additionally, they found that acidic HMO induce IFN-γ from ex vivo stimulated human cord blood mononuclear cells [45]. Isolated HMO enhanced proliferation of PBMC stimulated with a T-cell mitogen, phytohemagglutinin (PHA), and sialylated HMO enhanced proliferation of PBMC stimulated with the B-cell mitogen LPS [36]. In contrast, 2’ FL inhibited proliferation of unstimulated PBMC cultured for 3 days. Thus, the response to HMO may depend on the state of the infant. In the unstimulated state, HMO dampen proliferation, whereas HMO enhance proliferation in response to a mitogenic stimuli.
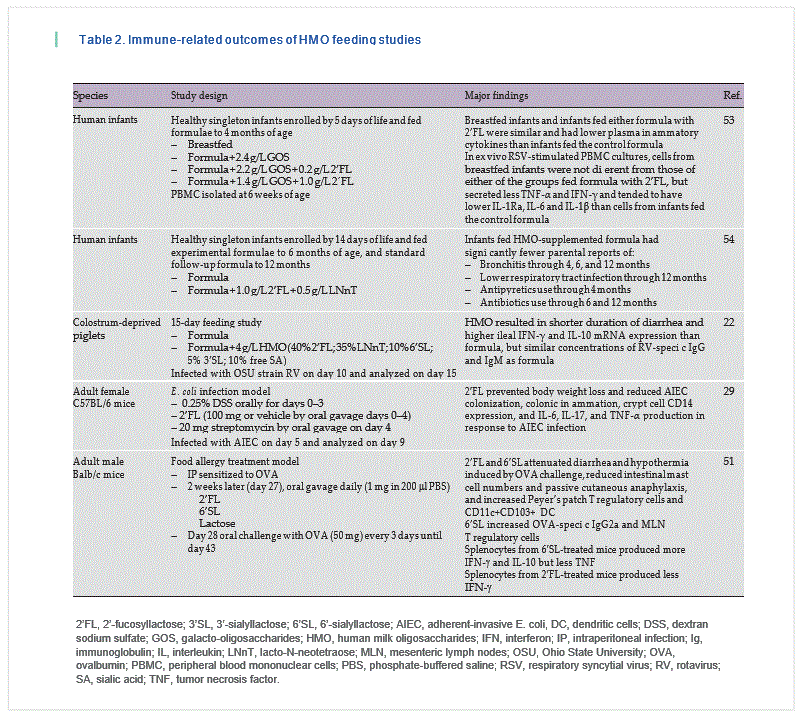
To date, very few studies have fed HMO and analyzed immune outcomes [22, 29, 30, 51–54] ( Table 2 ). Piglets [55] have been fed 2’ FL, but only growth and toxicological outcomes were reported. A recently published paper described immune outcomes in human infants fed formula containing 2.4 g/L GOS, 2.2 g/L GOS + 0.2 g/L 2’ FL, or 1.4 g/L GOS + 1.0 g/L 2’FL compared to a breastfed reference control [53]. Infants were fed the formula from 5 days to 4 months of age, and blood samples were obtained at 6 weeks of age for cytokine analysis, immune cell phenotyping, and ex vivo stimulation of isolated PBMC. Breastfed infants and infants fed either formula with 2’ FL were similar and had lower plasma inflammatory cytokines than infants fed the control formula. In addition, cytokine secretion by PBMC from breastfed infants and infants fed either 2’ FL-containing formula that were stimulated ex vivo with respiratory syncytial virus was similar and secreted less tumor necrosis factor-α and interferon-γ and tended to have lower IL-1Ra, IL-6, and IL-1β than cells from infants fed the control formula [53].
Another recent study in human infants evaluated the effect of formula supplemented with both 2’ FL (1.0 g/L) and LNnT (0.5 g/L) compared to an unsupplemented formula. Infants were fed the formulae from 14 days to 6 months of age, after which they were switched to a stan-dard follow-on formula and followed until 12 months of age. Infants fed the HMO- supplemented formula had significantly fewer parental reports (p = 0.004–0.047) of: bronchitis through 4 months (2.3 vs. 12.6%), 6 months (6.8 vs. 21.8%), and 12 months (10.2 vs. 27.6%); lower respiratory tract infection (AE cluster) through 12 months (19.3 vs. 34.5%); antipyretics use through 4 months (15.9 vs. 29.9%); and antibiotics use through 6 months (34.1 vs. 49.4%) and 12 months (42.0 vs. 60.9%) than those fed the standard formula [54].
Several studies in animal models support the reduced incidence of infection in human infants fed formula with HMO. In mice infected with Escherichia coli , once daily oral gavage with 100 mg, 2’ FL prevented body weight loss and reduced colonization with adherent-invasive E. coli , colonic inflammation, crypt cell CD14 expression, as well as IL-6, IL-17, and tumor necrosis factor-α production in response to adherent-invasive E. coli infection compared to mice treated with vehicle [29]. Mice fed 2’ FL and subjected to ileocecal resection gained more weight and had greater crypt depth and villus height at the site of transection than nonsupplemented mice [30]. The 2’ FL-fed mice also had upregulated mucosal immune response genes in the distal small bowel [30]. The studies where pigs and human infants were fed the HMO have focused on 2’ FL, which is readily available in large quantities at reasonable cost, and fucosylated oligosaccharides have been shown to feed specific beneficial classes of bacteria during intestinal inflammatory events [56]. Given what is known about the effects of other HMO, these compounds also should be used in feeding studies when available in sufficient quantities.
Only 1 in vivo study used a complex mixture of HMO and assessed immune outcomes. In that report, neonatal pigs fed a diet containing 4 g/L HMO, consisting of 40% 2’ FL, 10% 6’-sialyllactose (6’ SL), 35% lacto-N-neote-traose (LNnT), 5%, 3‘-sialyllactose (3’ SL), and 10% free sialic acid, had a reduced duration of diarrhea, in response to RV infection to 48.8 ± 9.8 h versus 80.6 ± 4.5 h in pigs fed nonsupplemented formula [22]. Ileal tissue from the pigs fed HMO contained greater IFN-γ (produced by Th1 cells) and IL-10 (an anti-inflammatory cytokine) mRNA than that from pigs fed formula [22].
In a mouse model of food allergy, 2’ FL and 6’ SL administered via oral gavage reduced symptoms in mice sensitized to ovalbumin, an egg protein [51]. Specifically, oval-bumin-stimulated splenocytes from mice treated with 6’SL produced more IL-10 and less IFN-γ than those from untreated mice. Furthermore, 2’ FL- or 6’ SL-treated mice had more regulatory immune cells in their intestinal immune tissues than untreated mice. Interestingly, neither 2’ FL nor 6’SL affected intestinal T regulatory cells when administered to nonsensitized mice [51]. This exemplifies the necessity of identifying an appropriate challenge model to assess the effects of dietary compounds on the immune system. In mice, the milk oligosaccharides LNFP III and LNnT are
Th2-biasing and suppress Th1 responses [57]. Recently, it has been reported that human infants who were fed human milk with low LNFP III concentrations (<60 μ M ) were 6.7-times (95% CI 2.0–22) more likely to become affected with cow’s milk allergy when compared to infants receiving milk with high LNFP III concentrations [58] .
Another approach using knockout mice showed that SL-containing compounds can directly affect gastrointestinal mucosal immunity [52, 59]. In one study, the presence of 3’SL in the milk increased the number of immune cells infiltrating the gut in IL-10 null mice [52]. Furthermore, supplementation with 3’SL increased colitis severity in newborn IL-10 and St3gal4 (the enzyme that synthesizes 3’SL) null mice, and cross-fostering wildtype mice to deficient dams reduced colitis severity. One caveat of this work is that it was conducted in the absence of endogenous IL-10 production, whereas other in vivo studies have demonstrated that some HMO increase intestinal IL-10 [22, 51]. 3’ SL is a product of several pathogenic bacteria [60] and the conformation (α2,3-link between sialic acid and galactose) on pathogenic bacteria and in human milk is the same. 3’ SL is recognized by DC and generates an immune response through the TLR4 signaling pathway [61]. These results suggest that the presence of 3’ SL increases the inflammatory response through direct effects on DC. When TLR4 was absent, 3’ SL was less effective at inducing DC activation. However, those DC also demonstrated a minimal increase in CD40 expression suggesting that at least one other 3’ SL-sensing mechanism, albeit much less efficient than the TLR4 pathway, exists on DC. TLR4 is the receptor for E. coli LPS. Another link between 3’ SL and TLR4 is ex-plained in a newer paper, where it is demonstrated that 3’ SL stimulates the proliferation of the intestinal E. coli population and that this overgrowth of E. coli is responsible for exacerbation of dextran sulfate sodium colitis through release of proinflammatory cytokines from intestinal DC [62].
These examples demonstrate the complexity of the relationships between oligosaccharides, the gut bacteria and the immune system.
Conclusion
The rich diversity of HMO has the potential to modulate both innate and adaptive neonatal immunity. Findings from in vitro experiments and animal models show that HMO directly interact with gastrointestinal epithelial cells as well as with mucosal and systemic immune cells to modulate immune function. HMO also beneficially shape the microbiome of the breastfed infant. The increased availability of HMO from commercial sources as well as accumulating evidence demonstrating that formula supplemented with HMO is safe and may confer benefits for human infants have led to the recent addition of 2’ FL alone or in combination with LNnT to infant formulae. In addition, due to their beneficial effects on immune function and host defense, HMO may also be beneficial for other segments of the population who are immune compromised or at high risk for infection. There are limited studies in which animals or humans have been fed HMO. Additionally, few studies have assessed the effects of feeding complex mixtures of HMO on the immune response. Thus, future research is needed to delineate mechanisms and to fully realize the potential for HMO to benefit infant immune function.
References
1 Levy O, Wynn JL: A prime time for trained immunity: innate immune memory in new-borns and infants. Neonatology 2014 ; 105: 136–141.
2 Walker WA, Iyengar RS: Breast milk, microbiota, and intestinal immune homeostasis. Pediatr Res 2015; 77: 220–228.
3 Andreas NJ, Kampmann B, Mehring Le Doare K: Human breast milk: a review on its composition and bioactivity. Early Hum Dev 2015; 91: 629–635.
4 Turfkruyer M, Verhasselt V: Breast milk and its impact on maturation of the neonatal immune system. Curr Opin Infect Dis 2015; 28: 199–206.
5 Donovan SM: Role of human milk components in gastrointestinal development: current knowledge and future needs. J Pediatr 2006; 149:S49–S61.
6 American Academy of Pediatrics, Section on Breastfeeding: Breastfeeding and the use of human milk. Pediatrics 2012; 129;e827–e841.
7 Horta BL, Victora CG: Long-term Effects of Breastfeeding. Geneva, World Health Organization, 2013. http://apps.who.int/ iris/bitst ream/10665/79198/1/9789241505307_eng.pdf (accessed September 15, 2016).
8 Li M, Wang M, Donovan SM: Early develop ment of the gut microbiome and immune-mediated childhood disorders. Semin Reprod Med 2014; 32: 74–86.
9 Wang M, Monaco MH, Donovan SM: Impact of early gut microbiota on immune and metabolic development and function. Semin Fetal Neonat Med 2016, Epub ahead of print.
10 Smilowitz JT, Lebrilla CB, Mills DA, German JB, Freeman SL: Breast milk oligosaccharides: structure-function relationships in the neonate. Annu Rev Nutr 2014 ; 34: 143–169.
11 Kunz C, Meyer C, Collado MC, Geiger L, García-Mantrana I, Bertua-Ríos B, Martínez- Costa C, Borsch C, Rudloff S: Influence of gestational age, secretor and Lewis blood group status on the oligosaccharide content of human milk. J Pediatr Gastroen-terol Nutr 2016, Epub ahead of print.
12 Thurl S, Munzert M, Henker J, Boehm G, Müller-Werner B, Jelinek J, Stahl B: Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr 2010; 104: 1261–1271.
13 Martín-Sosa S, Martín MJ, García-Pardo LA, Hueso P: Sialyloligosaccharides in hu-man and bovine milk and in infant formulas: variations with the progression of lactation. J Dairy Sci 2003; 86: 52–59.
14 Newburg DS, He Y: Neonatal gut microbiota and human milk glycans cooperate to attenuate infection and inflammation. Clin Obstet Gynecol 2015; 58: 814–826.
15 Kulinich A, Liu L: Human milk oligosaccharides: the role in the fine-tuning of innate immune responses. Carbohydr Res 2016 ; 432: 62–70.
16 Lewis ZT, Totten SM, Smilowitz JT, Popovic M, Parker E, Lemay DG, Van Tassell ML, Miller MJ, Jin YS, German JB, Lebrilla CB, Mills DA: Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome 2015; 3: 13.
17 Newburg DS, Ruiz-Palacios GM, Altaye M, Chaturvedi P, Meinzen-Derr J, Guerrero MDL, et al: Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology 2004; 14: 253–263.
18 Underwood MA, German JB, Lebrilla CB, Mills DA: Bifidobacterium longum subspecies infantis : champion colonizer of the infant gut. Pediatr Res 2015; 77: 229–235.
19 Garrido D, Barile D, Mills DA: A molecular basis for bifidobacterial enrichment in the infant gastrointestinal tract. Adv Nutr 2012; 3: 415S–421S.
20 Steenhout P, Sperisen P, Martin F-P, Sprenger N, Wernimont S, Pecquet S, Berger B: Term infant formula supplemented with human milk oligosaccharides (2’ fucosyllactose and lacto-N-neotetraose) shifts stool microbiota and metabolic signatures closer to that of breastfed infants. FASEB J 2016; 30 (suppl 1): 275.7.
21 Li M, Bauer LL, Chen X, Wang M, Kuhlen-schmidt TB, Kuhlenschmidt MS, Fahey GC Jr, Donovan SM: Microbial composition and in vitro fermentation patterns of human milk oligosaccharides and prebiotics differ between formula-fed and sow-reared piglets. J Nutr 2012; 142: 681–689.
22 Li M, Monaco MH, Wang M, Comstock SS, Kuhlenschmidt TB, Fahey GC Jr, Miller MJ, Kuhlenschmidt MS, Donovan SM: Human milk oligosaccharides shorten rotavirus-in-duceddiarrheaandmodulatepigletmucosalimmunityand colonic microbiota. ISME J 2014; 8: 1609–1620.
23 Goto Y, Kiyono H: Epithelial barrier: an interface for the cross-communication between gut flora and immune system. Immu-nol Rev 2012; 245: 147–163.
24 Sassone-Corsi M, Raffatellu M: No vacancy: how beneficial microbes cooperate with im-munityto provide colonization resistance to pathogens. J Immunol 2015; 194: 4081–4087.
25 Hester SN, Donovan SM: Individual and combined effects of nucleotides and human milk oligosaccharides on proliferation, apoptosis and necrosis in a human fetal intestinal cell line. Food Nutr Sci 2012 ; 3: 1567–1576.
26 Holscher HD, Davis SR, Tappenden KA: Human milk oligosaccharides influence maturation of human intestinal Caco-2Bbe and HT-29 cell lines. J Nutr 2014; 144: 586–591.
27 Bhatia S, Prabhu PN, Benefiel AC, Miller MJ, Chow J, Davis SR, Gaskins HR: Galacto-oligosaccharides may directly enhance intestinal barrier function through the modulation of goblet cells. Mol NutrFood Res 2015 ; 59: 566–573.
28 Lane JA, O’Callaghan J, Carrington SD, Hickey RM: Transcriptional response of HT-29 intestinal epithelial cells to human and bovine milk oligosaccharides. Br J Nutr 2013; 110: 2127–2137.
29 He Y, Liu S, Kling DE, Leone S, Lawlor NT, Huang Y, Feinberg SB, Hill DR, Newburg DS: The human milk oligosaccharide 2’-fu-cosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 2016 ; 65: 33–46.
30 Mezoff EA, Hawkins JA, Ollberding NJ, Karns R, Morrow AL, Helmrath MA: The human milk oligosaccharide 2’-fucosyllac-tose augments the adaptive response to extensive intestinal resection. Am J Physiol Gastrointest Liver Physiol 2016 ; 310:G427–G438.
31 Wickramasinghe S, Pacheco AR, Lemay DG, Mills DA: Bifidobacteria grown on human milk oligosaccharides downregulate the expression of inflammation-related genes in Caco-2 cells. BMC Microbiol 2015; 15: 172.
32 Marcobal A, Sonnenburg JL: Human milk oligosaccharide consumption by intestinal microbiota. Clin Microbiol Infect 2012; 18(suppl 4):12–15.
33 Kavanaugh D, O’Callaghan J, Kilcoyne M, Kane M, Joshi L, Hickey RM: The intestinal glycome and its modulation by diet and nutrition. Nutr Rev 2015; 73: 359–375
34 Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL: Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol 2008; 295: 1025–1034.
35 Macpherson AJ, Geuking MB, McCoy KD: Immune responses that adapt the intestinal mucosa to commensal intestinal bacteria. Immunology 2005; 115: 153–162.
36 Comstock SS, Wang M, Hester SN, Li M, Donovan SM: Select human milk oligosac- charides directly modulate peripheral blood mononuclear cells isolated from 10-d-old pigs. Br J Nutr 2014; 111: 819–828.
37 Goehring KC, Kennedy AD, Prieto PA, Buck RH: Direct evidence for the presence of human milk oligosaccharides in the circula-tion of breastfed infants. PLoS One 2014 ; 9: e101692.
38 Marriage BJ, Buck RH, Goehring KC, Oliver JS, Williams JA: Infants fed a lower calorie formula with 2’-fucosyllactose (2’ FL) show growth and 2’FL uptake like breast-fed infants. J Pediatr Gastroenterol Nutr 2015 ; 61: 649–658.
39. Ruhaak LR, Stroble C, Underwood MA, LebrillaCB: Detection of milk oligosaccharides in plasma of infants. Anal Bioanal Chem 2014; 406: 5775–5784.
40. Bode L, Kunz C, Muhly-Reinholz M, Mayer K, Seeger W, Rudloff S: Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb Haemost 2004; 92: 1402–1410.
41. Bode L, Rudloff S, Kunz C, Strobel S, Klein N: Human milk oligosaccharides reduce platelet-neutrophil complex formation leading to a decrease in neutrophil beta 2 integrin expression. J Leukoc Biol 2004; 76: 820–826.
42. Rabinovich GA, Croci DO: Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity 2012; 36: 322–335.
43. Schnaar RL: Glycans and glycan-binding proteins in immune regulation: a concise introduction to glycobiology for the allergist. J Allergy Clin Immunol 2015; 135: 609–615.
44. Geijtenbeek TB, van Vliet SJ, Engering A, ‘t Hart BA, van Kooyk Y: Self- and nonselfrecognition by C-type lectins on dendritic cells. Annu Rev Immunol 2004; 22: 33–54.
45. Koning N, Kessen SF, Van Der Voorn JP, Appelmelk BJ, Jeurink PV, Knippels LM, Garssen J, Van Kooyk Y: Human milk blocks DCSIGN-pathogen interaction via MUC1. Front Immunol 2015; 6: 112.
46. Macauley MS, Crocker PR, Paulson JC: Siglec- mediated regulation of immune cell function in disease. Nat Rev Immunol 2014; 14: 653–666.
47. Stephenson HN, Mills DC, Jones H, Milioris E, Copland A, Dorrell N, Wren BW, Crocker PR, Escors D, Bajaj-Elliott M: Pseudaminic acid on Campylobacter jejuni flagella modulates dendritic cell IL-10 expression via Siglec-10 receptor: a novel flagellin-host interaction. J Infect Dis 2014; 210: 1487–1498.
48. Earl LA, Bi S, Baum LG: N- and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death. J Biol Chem 2010; 285: 2232–2244.
49. Hester SN, Chen X, Li M, Monaco MH, Comstock SS, Kuhlenschmidt TB, Kuhlenschmidt MS, Donovan SM: Human milk oligosaccharides inhibit rotavirus infectivity in vitro and in acutely infected piglets. Br J Nutr 2013; 110: 1233–1242.
50. Luhn K, Wild MK: Human deficiencies of fucosylation and sialylation affecting selectin ligands. Semin Immunopathol 2012; 34: 383–399.
51. Castillo-Courtade L, Han S, Lee S, Mian FM, Buck R, Forsythe P: Attenuation of food allergy symptoms following treatment with human milk oligosaccharides in a mouse model. Allergy 2015; 70: 1091–1102.
52. Kurakevich E, Hennet T, Hausmann M, Rogler G, Borsig L: Milk oligosaccharide sialyl(a2,3)lactose activates intestinal CD11c+ cells through TLR4. Proc Natl Acad Sci USA 2013; 110: 17444–17449.
53. Goerhring KC, Marriage BJ, Oliver JS, Wilder JA, Barrett EG, Buck RH: Similar to those who are breastfed, infants fed a formula containing 2’-fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. J Nutr 2016, DOI: 10.3945/jn.116.236919.
54. Puccio G, Alliet P, Cajozzo C, Janssens E, Corsello G, Wernimont S, Egli D, Gosoniu L, Sprenger N, Steenhout P: Effects of infant formula with human milk oligosaccharides on growth and morbidity: a randomized multicenter trial. JPGN, in press.
55. Hanlon PR, Thorsrud BA: A 3-week preclinical study of 2’-fucosyllactose in farm piglets. Food Chem Toxicol 2014; 74: 343–348.
56. Kashyap PC, Marcobal A, Ursell LK, Smits SA, Sonnenburg ED, Costello EK, Higginbottom SK, Domino SE, Holmes SP, Relman DA, Knight R, Gordon JI, Sonnenburg JI: Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc Natl Acad Sci USA 2013; 110: 17059–17064.
57. Okano M, Satoskar AR, Nishizaki K, Harn DA Jr: Lacto-N-fucopentaose III found on Schistosoma mansoni egg antigens functions as adjuvant for proteins by inducing Th2-type response. J Immunol 2001; 167: 442–450.
58. Seppo AE, Autran CA, Bode L, Järvinen KM: Human milk oligosaccharides and development of cow’s milk allergy in infants. J Allergy Clin Immunol 2016, Epub ahead of print.
59. Huang YL, Chassard C, Hausmann M, von Itzstein M, Hennet T: Sialic acid catabolism drives intestinal inflammation and microbial dysbiosis in mice. Nat Commun 2015; 6: 8141.
60. Severi E, Hood DW, Thomas GH: Sialic acid utilization by bacterial pathogens. Microbiology 2007; 153: 2817–2822.
61. Audry M, Jeanneau C, Imberty A, Harduin-Lepers A, Delannoy P, Breton C: Current trends in the structure- activity relationships of sialyltransferases. Glycobiology 2011; 21:716–726.
62. Kuijf ML, Samsom JN, van Rijs W, Bax M, Huizinga R, Heikema AP, van Doorn PA, van Belkum A, van Kooyk Y, Burgers PC, Luider TM, Endtz HP, Nieuwenhuis EE, Jacobs BC: TLR4-mediated sensing of Campylobacter jejuni by dendritic cells is determined by sialylation. J Immunol 2010; 185: 748–755.