Complementary Feeding, Infant Growth, and Obesity Risk: Timing, Composition and Mode of Feeding
The complementary feeding (CF) period is a short transitional period from breastfeeding and formula feeding to family foods. Timing, quantity, and quality are implied to impact growth and obesity risk through changes in energy and nutrient intakes due to flavor shaping and other mechanisms. CF is influenced by concurrent and previous feeding (breastfeeding and formula feeding) which also impact growth. All aspects of CF timing, quantity, and quality are also influenced by infants (e.g., genetics) and environmental factors (e.g., socioeconomic status and parental feeding style) that partly interact (Fig. 1).
We analyzed data of monthly food diaries of more than 1,000 children from 5 European countries in the first 2 years of life with similar infant feeding recommendations, which were collected as part of the prospective European Childhood Obesity Project (CHOP Study) [1]. While the recommendations state that CF should not start before 6 months of age (World Health Organization) or between 4 and 6 months of age (e.g., ESPGHAN [2]), timing varies considerably together with the quantity and quality of complementary foods [1, 3, 4]. Timing depended on previous and concurrent feeding (breastfeeding and formula feeding), country of residence, socioeconomic status, smoking in pregnancy, birth weight, and other factors [4]. Formula-fed children, for instance, start approximately 2 weeks earlier in Europe than breastfed children and in almost 40% at or before 4 months of age [1]. While introduction of solids between 4 and 6 months or after 6 months does not seem to impact growth and later obesity risk [4], solids before 4 months of age increase the risk: odds ratio for obesity 1.33, 95% confidence interval (CI) 1.07–1.64 [5]. There are indications that this is especially problematic for formula-fed children. For these children, complementary foods seem to add energy to their diet [4]. In a comparison of children eating solids with those being exclusively formula fed, we have shown that solid eaters had generally high energy intakes, varying between +96 kcal/day (95% CI 64–128 kcal/day) at 3 months of age and +87 kcal/day (95% CI 42–133 kcal/day) at 6 months of age [4].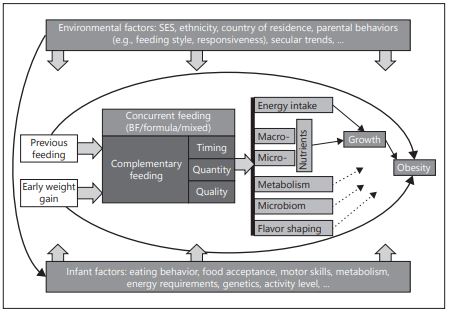
Fig. 1. Infants innate and environmental factors affecting later obesity risk. BF, breastfeeding; SES, socioeconomic status
During the CF period, fat intake decreases, and protein and carbohydrate intakes increase. Protein intake often exceeds European recommendations from 9 months onwards [4]. However, while there are indications that high dairy protein intakes during CF play a role in weight gain, randomized controlled trials are lacking. Also, the role of increasing carbohydrate intake on metabolism and flavor shaping and later growth needs to be further evaluated to draw any conclusions. Depending on the region, commercial infant foods and self-cooked products have a different share on infant intakes; both generally show differences in nutrient content and variety, with commercial products having generally a lower variety and higher carbohydrate content.
Only few studies related the mode of feeding (e.g., responsive feeding or baby-led feeding) during CF to growth. In general, these findings are not conclusive and studies are still ongoing. There are indications that responsive feeding – as recommended by WHO guidelines – is beneficial for favorable growth. In summary, no benefit or disadvantage has been shown in terms of growth to start complementary foods either between 4 and 6 or after 6 months of age, but early introduction of complementary foods before 4 months of age should be avoided, supporting current recommendations [6]. The impact of CF quality on short-term growth and later obesity risk has to be elucidated further.
Acknowledgments
The authors’ work is financially supported by the European Commission, projects EarlyNutrition (FP7–289346), DynaHEALTH (H2020– 633595), and LifeCycle (H2020-SC1–2016-RTD), and the European Research Council Advanced Grant META-GROWTH (ERC-2012-AdG 322605).
References
- Schiess S, Grote V, Scaglioni S, et al: Introduction of complementary feeding in 5 European countries. J Pediatr Gastroenterol Nutr 2010;50:92–98.
- Fewtrell M, Bronsky J, Campoy C, et al: Complementary feeding: a position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J Pediat Gastroenterol Nutr 2017;64:119–132.
- Schiess SA, Grote V, Scaglioni S, et al: Intake of energy providing liquids during the first year of life in five European countries. Clin Nutr 2010;29:726–732.
- Grote V, Schiess SA, Closa-Monasterolo R, et al: The introduction of solid food and growth in the first 2 y of life in formula-fed children: analysis of data from a European cohort study. Am J Clin Nutr 2011;94:1785S–1793S.
- Damianidi L, Gruszfeld D, Verduci E, et al: Protein intakes and their nutritional sources during the first 2 years of life: secondary data evaluation from the European Childhood Obesity Project. Eur J Clin Nutr 2016;70:1291–1297.
- Wang J, Wu Y, Xiong G, et al: Introduction of complementary feeding before 4 months of age increases the risk of childhood overweight or obesity: a meta-analysis of prospective cohort studies. Nutr Res 2016;36:759–770.
Abstract
The complementary feeding period is a short transitional period from breastfeeding and formula feeding to family foods. Timing, quantity, and quality are implied to impact growth and obesity risk. We summarized the literature and analyzed data of monthly 3-day food diaries of >1,000 children from 5 European countries in the first 2 years of life, which were collected as part of the prospective European Childhood Obesity Project (CHOP Study). Formula-fed children started complementary food approximately 2 weeks earlier than breastfed children, and almost 40% of them at or before 4 months of age. While in- troduction of solids between 4 and 6 months or after 6 months does not seem to impact growth and later obesity risk, solids before 4 months of age increased the risk. There are indications that this is especially problematic for formula-fed children. During the comple- mentary feeding period, fat intake decreases, and protein and carbohydrate intakes in- crease. Protein intake often exceeds European recommendations from 9 months onwards. However, the role of macronutrients during complementary feeding in growth and me- tabolism needs further clarification. Findings on the role of responsive feeding or baby-led feeding during complementary feeding in growth are not conclusive. In summary, while introduction of complementary foods before 4 months of age should be avoided, the im- pact of the quality of complementary food on short-term growth and later obesity risk has to be elucidated further.
Introduction
The complementary feeding period is a dietary transitional period from solely milk feedings to a diet with milk feedings and family foods usually ending in the second year of life. Studying the influence of complementary feeding on obesity risk requires considering issues around timing of introduction to solid foods, as well as the quantity and quality of complementary foods (CF).
Timing of introduction to solid foods is considered of importance, as growth dynamics change dramatically during the first year of life, and inappropriate nutritional intake can change infant growth rates, which have been identified as an important risk factor for later obesity [1]. Quantity and quality of CF influence energy as well as macro- and micronutrient intakes, which both influence early growth [2, 3]. These aspects are in turn related to the concurrent feeding, previous feeding (breastfeeding or formula feeding), early weight gain, and other environmental and infant intrinsic factors [4, 5]. Variation in complementary feeding could lead, for instance, to metabolic or microbiome imprinting and growth differences that predispose to obesity. Furthermore, flavor shaping during the complementary feeding period influence later food preferences that in turn increase the obesity risk [6]. To detangle the qualitative impact of CF and family foods is generally a difficult task, and rigorous study designs are required to answer this question. Figure 1 outlines the major innate and environmental factors and their complex relationship with complementary feeding towards an increased risk of later obesity.
This paper focuses on the impact of complementary feeding and feeding mode on growth and later obesity risk in Europe. A further objective of this pa- per is to discuss which nutritional changes take place in the complementary feeding period, and which complementary feeding practices and related nutritional changes impact infant weight gain and overall obesity risk. We will use results from the EU Childhood Obesity Project (CHOP Study) to illustrate several of these aspects.
EU Childhood Obesity Project: Methodological Background Information
The prospective European CHOP Study analyzed complementary feeding data from monthly food diaries of more than 1,000 children from 5 European countries over the first 2 years of life with about 10,000 food diaries [7] (Fig. 2). This randomized intervention trial is studying the risk of obesity in a birth cohort that had randomized exposure to infant formulas with 2 different levels of protein.
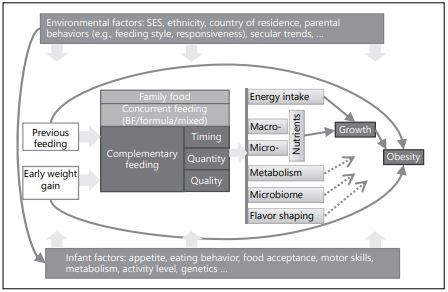
Fig. 1. Infants innate and environmental factors affecting later obesity risk. BF, breast- feeding; SES, socioeconomic status.
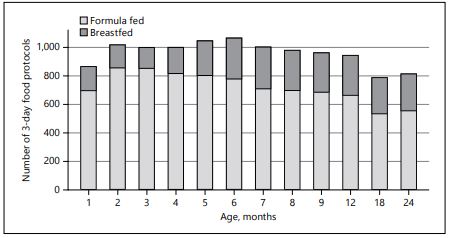
Fig. 2. Total 3-day food diaries collected in the CHOP study by primary milk feeding over the first 2 years of life.
Infants were recruited between 2002 and 2004 between birth and 8 weeks of life and were healthy, singleton newborns from uncomplicated pregnancies. Written informed consent from parents and approval from local ethics commit- tees were obtained. If parents chose formula feeding, infants were randomized to receive 1 of 2 experimental infant formulas: a conventional infant formula with high protein content or an infant formula with reduced protein content. A group of breastfed infants was followed as a reference group.
Dietary data were collected using 3-day weighted food diaries. Parents were instructed to record food intake on 2 weekdays and 1 weekend day every month for the first 9 months of life, and at the ages of 12, 18, and 24 months. Using a digital scale and detailed instructions on how to weigh and record foods on the dietary protocols, parents recorded their infants’ daily dietary intake.
A standard operating procedure (SOP) manual and software system (SOP- System) were created in order to standardize the recording of infant formula dilution and to minimize data entry errors [8, 9].
Timing of Solid Food Introduction and Risk for Obesity
The current recommendation of the World Health Organization is to start CF not before 6 months of age [10, 11]. This is largely motivated by the observation that early introduction of CF in developing countries is associated with increased risks for infection, growth faltering, and undernutrition. In many countries, to- day, obesity is a quantitatively greater public health concern than undernutrition. The conclusions of the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) and the European Food Safety Authority (EFSA) agree that solid foods can be safely introduced between 4 and 6 months of age in infants in Europe [12, 13]. The actual timing of introduction of CF varies considerably between countries [14, 15]. The timing of introduction is associated with previous and concurrent human milk or infant formula feed- ings, country of residence, socioeconomic status, smoking in pregnancy, birth weight, and other factors [7, 16]. The association between early solid food introduction in infancy and obesity in early childhood may also be modified by the type of primary milk feeding [17].
On average, in Europe, formula-fed infants start solid foods approximately 2 weeks earlier than breastfed infants, and almost 40% of infants start solid foods at or before 4 months of age [15, 16]. Evaluation of dietary data from the CHOP study shows that infants were introduced to CF starting from early in- fancy, with a sharp increase in CF between 4 and 6 months of age. By 6 months of age, almost all infants in the cohort had started solid foods [7]. The introduction of solids between 4 and 6 months or after 6 months showed no differential impact on growth and later obesity risk, but introduction of solids before 4 months of age was associated with an increased obesity risk (odds ratio, OR, 1.33, 95% confidence interval, CI, 1.07–1.64) [15]. In this study, there were in- dications that this is especially problematic for formula-fed infants, where CF seems to add additional energy to their diets rather than displace calories from infant formula.
The results of studies analyzing the role of solid food introduction have been consolidated in recent reviews and meta-analyses. In 2010, the first review used data from over 34,000 participants for analysis but found no clear association between the age of introduction and obesity risk [18]. In 2013, a further systematic review included 23 studies and showed that there is some evidence that introducing CF before 4 months of age increases the risk for obesity [19]. Other than this finding, there was no consistent association of the age at first introduction of CF with the risk of overweight and obesity [19].
A narrative review in 2015 called for more and better-quality evidence in order to inform guidelines on the timing of introduction of solid foods [20]. It stated that early introduction, defined as introduction of CF before 4 months of age, increases the risk for childhood obesity, but that there is no evidence that childhood obesity risk is increased with the introduction of solid foods at 4–6 months of age compared to introduction at age 6 months [20].
In 2016, a meta-analysis of 13 prospective cohort studies evaluated the association between the age at introduction of complementary feeding and the risk of overweight or obesity during childhood [21]. The analysis included 63,605 participants with 11,900 incident overweight cases and 56,136 participants with 3,246 incident obese cases. Results revealed that introducing CF before 4 months compared to 4–6 months of age was associated with an increased risk (relative risk, RR, 1.18; 95% CI, 1.06–1.31) of being overweight or obese (RR, 1.33; 95% CI, 1.07–1.64) during childhood. However, no significant relationship was ob- served between delaying introduction of CF until after 6 months of age [21]. In summary, studies indicate that early introduction to solid foods (before 4 months of age) results in an increased risk for childhood overweight.
Quality and Quantity of Complementary Foods and Obesity Risk
The quantity and quality of CF varies considerably between countries. During this transitional period, the total daily fat intake decreases while total daily protein and carbohydrate intakes increase [3, 22]. While it has been shown in a randomized trial that higher protein intake during formula feeding in the first year of life leads to a higher obesity risk in school age [23], there are some indications that too much dairy protein during the complementary feeding period might also lead to a higher obesity risk later in life [24].
In 746 formula-fed infants from the CHOP study, dairy protein from infant formula was the main source of protein in the first 2 years of life. Average protein intake exceeded European recommendations from 9 months of age on- wards, and meat protein added an increasing proportion of protein intake [25]. Depending on the country of residence, commercial CF (CCF) and home- made CF contribute differently to dietary intakes. Significant proportions of daily energy from CF consumed are CCF, and intakes of these foods vary by infant age. Homemade CF and CCF generally show differences in nutrient content and variety, with some indication that commercial infant foods tend to have higher carbohydrate contents [26–28]. In general, these findings are not conclusive, and their impact on growth and obesity has not been shown.
In 2013, Pearce et al. [19] published a systematic review outlining the current evidence on the types of CF which are associated with a higher risk of childhood obesity. Ten studies were identified with complementary feeding data and body mass index (BMI) in later life (>4 years). The review did not find any data that showed an association with fat and carbohydrate intakes and obesity. There were also no clear data on particular types of CF and obesity risk. They concluded that high intakes of energy and protein, particularly dairy protein, in infancy could be associated with childhood obesity but that further research is needed to establish the nature of the relationship.
The Avon Longitudinal Study of Parents and Children (ALSPAC) in the UK investigated if feeding of high volumes of cows’ milk (>600 mL) at 8 months of age was associated with faster weight and height gain compared to breastfeeding. They found high volumes of cows’ milk in late infancy in formula-fed infants may have a persisting effect on body composition throughout childhood (up to 10 years of age) [29]. This finding is in line with other findings of higher protein intake during the complementary feeding period. However, high intake of cows’ milk is generally not recommended during the first year of life [12].
Energy-Providing Liquids and Obesity Risk
Energy-providing liquids (EPL) have been investigated in recent years with regard to their impact on childhood obesity. Sugar-sweetened beverages (SSB) are one type of EPL where sugar is added either during industrial processing or at home. Several studies on the effects of SSB alone, or together with other EPL, or specific associations of particular EPL, such as fruit juice, with overweight and obesity have been published.
A recent study examining juice intake in infancy and its relationship with obesity found that higher juice intake at 1 year of age was associated with higher juice intake, SSB intake, and BMI z-score in early and mid-childhood [30]. An- other study found SSB intake during infancy to significantly increase the likeli-
hood of consuming these beverages more than once per day at 6 years of age [31]. A study from the same research group confirmed that children who consumed SSB during infancy had higher odds of obesity at 6 years than non-SSB consumers and that SSB consumption during infancy may be a risk factor for obesity [32].
A recent systematic review of systematic reviews on exposures in infancy and subsequent risk of obesity states that there is no consistent evidence to suggest an association between SSB intake in early childhood and long-term overweight and obesity [33]. However, this review included only very few studies during the complementary feeding period.
In the CHOP Study, an analysis of the intake of EPL in the first 10 months of life showed that intake varies by the initial milk feeding type and infant age [34]. EPL were introduced very early, with some infants receiving EPL already from birth. EPL intakes added excess energy to infant diets. Due to the generally low intakes, effects on growth have not been evaluated.
Feeding Mode
Only a few studies have related the mode of feeding such as “baby-led weaning,” “prolonged bottle feeding,” and “responsive feeding” on infant growth and obesity risk. “Baby-led weaning” is a relatively new concept where infants are encouraged to begin with solid foods around 6 months of age. Using this feeding mode, infants only self-feed pieces of food that they can grab with the hand from the very beginning of the complementary feeding period, and no spoon feeding is accept- ed. Infants are offered similar foods as the family but as finger food. Skeptics of the baby-led weaning feeding mode raise concern regarding an increased risk of undernutrition, since an infant’s restricted motor control impairs their ability to self-feed with sufficient calories or the desired variety of foods that provides ad- equate amounts of critical micronutrients such as iron. Baby-led weaning is driven by a hypothesis of improved infant self-control and self-regulation of dietary intake without directing parental involvement at feeding times [35, 36].
One recently published, randomized, controlled trial on baby-led weaning in a cohort in New Zealand showed that the baby-led weaning approach did not result in significantly lower BMI at 12 or 24 months of age compared to spoon feeding [37]. However, the authors reported that the different feeding modes did affect infant acceptability, satiety, and enjoyment of foods [37]. In conclusion, the concept of baby-led weaning raises important questions as to the develop- mental readiness of infants to carry out self-feeding. Furthermore, the potentially increased risk of choking is not yet answered [38].
In infants fed with baby-led weaning, the amounts of CF consumed increase at a slower pace and later than in infants who also receive spoon feeding, and hence they receive a larger proportion of milk feeding for longer. An investigation into prolonged bottle use as a risk factor for later obesity was published by the United States Early Childhood Longitudinal Study, Birth Cohort (ECLS-B) in 2011. In 6,750 children, prolonged bottle use, especially during the night, un- til 2 years of age was associated with obesity at 5.5 years of age [39]. This observation reiterates the general recommendation to stop bottle feeding in the second year of life and shift to offering liquids from a cup [12].
Responsive feeding is when parents are sensitive to and respect the hunger and satiety cues when feeding young infants and assist older children to feed themselves [40]. There are indications that responsive feeding as recommended by the WHO guidelines [41] is beneficial for optimal infant growth. The concept behind responsive feeding is that feeding times are periods of learning and love, and parents are encouraged to talk to their infants and young children during feeding and to make eye contact.
The Intervention Nurses Start Infants Growing on Healthy Trajectories (IN- SIGHT) Study investigated whether a responsive feeding intervention affected infant dietary patterns in 291 infants in the United States [42]. While over 60% of infants had patterns low in fruits and vegetables or high in energy-dense foods, the responsive feeding intervention was associated with healthier dietary patterns [42]. There were no reported effects of the intervention itself on BMI, but food groups were associated with BMI at 2 years of age [42].
In Australia, a randomized controlled trial(NOURISH), evaluated a universal intervention commencing in infancy to provide anticipatory guidance to 698 first-time mothers on complementary feeding practices hypothesized to reduce childhood obesity risk [43]. During early childhood, intervention mothers re- ported less frequent use of nonresponsive feeding practices and more appropriate responses to food refusal. No statistically significant group effect was noted for BMI z-scores or overweight/obesity prevalence [43].
Conclusion
There is evidence that CF should not be introduced before 4 months of age due to an increased risk for obesity. This effect is more pronounced in formula-fed infants, whose daily energy intakes are increased through CF com- pared to breastfed infants, who seem to better self-regulate their energy in- takes from CF. According to current evidence, there are no known benefits or disadvantages to introducing CF either at 4–6 or after 6 months of age with respect to obesity risk in children in high-income countries such as European countries.
There is a need for more research on the impact of the quality of CF. EPL and SSB increase the risk for obesity and should be avoided. Excessive cows’ milk intake during the complementary feeding period should also be avoided. There is a need for more research to determine if high protein intake during complementary feeding, particularly dairy protein, increases the risk of later obesity. There is also a need to investigate the impact of the amounts and composition of simple and complex carbohydrates, glycemic indices, and sweet tasting CF on infant growth, food acceptance, and later obesity risk. One also needs to explore how different infant feeding patterns affect metabolic outcomes as well as metabolic programming effects of complementary feeding choices.
Responsive feeding seems to be beneficial for growth patterns and health. While a randomized controlled trial on baby-led weaning did not find significant differences in obesity risk between different baby-led and spoon-fed feeding modes, there is a need for more intervention trials to determine if baby-led weaning is nutritionally adequate, improves infant feeding practices overall, and has any measurable impact of the risk for childhood obesity.
Acknowledgments
The authors’ work is financially supported in part by the European Commission, project EarlyNutrition (FP7-289346), DynaHEALTH (H2020-633595), LifeCycle (H2020-SC1- 2016-RTD), and the European Research Council Advanced Grant META-GROWTH (ERC-2012-AdG 322605).
Disclosure Statement
- V.G.has nothing to declare beside the support of Nestec for the conference. All other authors declare no conflicts of interest.
References
- Monteiro PO, Victora CG: Rapid growth in infancy and childhood and obesity in later life – a systematic review. Obes Rev 2005;6: 143–154.
- Ong KK, Emmett PM, Noble S, et al: Dietary energy intake at the age of 4 months predicts postnatal weight gain and childhood body mass index. Pediatrics 2006;117:e503–e508.
- Koletzko B, von Kries R, Closa R, et al: Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clin- ical trial. Am J Clin Nutr 2009;89:1836–1845.
- Woo Baidal JA, Locks LM, Cheng ER, et al: Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med 2016;50:761–779.
- Young BE, Krebs NF: Complementary feed- ing: critical considerations to optimize growth, nutrition, and feeding behavior. Curr Pediatr Rep 2013;1:247–256.
- Mennella JA, Trabulsi JC: Complementary foods and flavor experiences: setting the foundation. Ann Nutr Metab 2012;60(suppl 2):40–50.
- Schiess S, Grote V, Scaglioni S, et al; Europe- an Childhood Obesity Project: Introduction of complementary feeding in 5 European countries. J Pediatr Gastroenterol Nutr 2010; 50:92–98.
- Verwied-Jorky S, Schiess S, Luque V, et al; European Childhood Obesity P: Methodol- ogy for longitudinal assessment of nutrient intake and dietary habits in early childhood in a transnational multicenter study. J Pediatr Gastroenterol Nutr 2011;52:96–102.
- Luque V, Escribano J, Mendez-Riera G, et al: Methodological approaches for dietary intake assessment in formula-fed infants. J Pediatr Gastroenterol Nutr 2013;56:320–327.
- World Health Organization: Complementary feeding: family foods for breastfed children: WHO/NHD/001, WHO/FCH/CAH/006. Ge- neva, WHO, 2000.
- World Health Organization: Guiding Prin- ciples for Feeding Non-Breastfed Children 6–24 Months of Age. Geneva, WHO, 2005.
- ESPGHAN Committee on Nutrition, Fewtrell M, Bronsky J, et al: Complementary feeding: a position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutri- tion. J Pediatr Gastroenterol Nutr 2017;64: 119–132.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA): Scientific opinion on the appropriate age for introduction of com- plementary feeding of infants. EFSA J 2009;7: 1423.
- Woo JG, Guerrero ML, Ruiz-Palacios GM, et al: Specific infant feeding practices do not consistently explain variation in anthropom- etry at age 1 year in urban United States, Mexico, and China cohorts. J Nutr 2013;143: 166–174.
- Grote V, Schiess SA, Closa-Monasterolo R, et al; European Childhood Obesity Trial Study Group: The introduction of solid food and growth in the first 2 y of life in formula-fed children: analysis of data from a European cohort study. Am J Clin Nutr 2011;94:1785S– 1793S.
- Fewtrell MS, Lucas A, Morgan JB: Factors associated with weaning in full term and pre- term infants. Arch Dis Child 2003;88:F296– F301.
- Huh SY, Rifas-Shiman SL, Taveras EM, et al: Timing of solid food introduction and risk of obesity in preschool-aged children. Pediatrics 2011;127:e544–e551.
- Moorcroft KE, Marshall JL, McCormick FM: Association between timing of introducing solid foods and obesity in infancy and child- hood: a systematic review. Matern Child Nutr 2011;7:3–26.
- Pearce J, Taylor MA, Langley-Evans SC: Tim- ing of the introduction of complementary feeding and risk of childhood obesity: a sys- tematic review. Int J Obes (Lond) 2013;37: 1295–1306.
- Daniels L, Mallan KM, Fildes A, Wilson J: The timing of solid introduction in an ‘obe- sogenic’ environment: a narrative review of the evidence and methodological issues. Aust NZ J Public Health 2015;39:366–373.
- Wang J, Wu Y, Xiong G, et al: Introduction of complementary feeding before 4 months of age increases the risk of childhood over- weight or obesity: a meta-analysis of prospec- tive cohort studies. Nutr Res 2016;36:759– 770.
- Ahluwalia N, Herrick KA, Rossen LM, et al: Usual nutrient intakes of US infants and tod- dlers generally meet or exceed dietary refer- ence intakes: findings from NHANES 2009– 2012. Am J Clin Nutr 2016;104:1167–1174.
- Weber M, Grote V, Closa-Monasterolo R, et al; European Childhood Obesity Trial Study Group: Lower protein content in infant for- mula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr 2014;99:1041–1051.
- Panjwani A, Heidkamp R: Complementary feeding interventions have a small but signifi- cant impact on linear and ponderal growth of children in low- and middle-income coun- tries: a systematic review and meta-analysis. J Nutr 2017;147:2169S–2178S.
- Damianidi L, Gruszfeld D, Verduci E, et al: Protein intakes and their nutritional sources during the first 2 years of life: secondary data evaluation from the European Childhood Obesity Project. Eur J Clin Nutr 2016;70: 1291–1297.
- Foterek K, Hilbig A, Alexy U: Associations between commercial complementary food consumption and fruit and vegetable intake in children. Results of the DONALD study. Appetite 2015;85:84–90.
- Mesch CM, Stimming M, Foterek K, et al: Food variety in commercial and homemade complementary meals for infants in Germa- ny. Market survey and dietary practice. Ap- petite 2014;76:113–119.
- Mok E, Vanstone CA, Gallo S, et al: Diet di- versity, growth and adiposity in healthy breastfed infants fed homemade complemen- tary foods. Int J Obes (Lond) 2017;41:776– 782.
- Hopkins D, Steer CD, Northstone K, Emmett PM: Effects on childhood body habitus of feeding large volumes of cow or formula milk compared with breastfeeding in the latterpart of infancy. Am J Clin Nutr 2015;102: 1096–1103.
- Sonneville KR, Long MW, Rifas-Shiman SL, et al: Juice and water intake in infancy and later beverage intake and adiposity: could juice be a gateway drink? Obesity (Silver Spring) 2015;23:170–176.
- Park S, Pan L, Sherry B, Li R: The association of sugar-sweetened beverage intake during infancy with sugar-sweetened beverage intake at 6 years of age. Pediatrics 2014;134(suppl 1):S56–S62.
- Pan LP, Li RW, Park SY, et al: A longitudinal analysis of sugar-sweetened beverage intake in infancy and obesity at 6 years. Pediatrics 2014;134:S29–S35.
- Patro-Golab B, Zalewski BM, Kolodziej M, et al: Nutritional interventions or exposures in infants and children aged up to 3 years and their effects on subsequent risk of over- weight, obesity and body fat: a systematic re- view of systematic reviews. Obes Rev 2016;17: 1245–1257.
- Schiess SA, Grote V, Scaglioni S, et al: Intake of energy providing liquids during the first year of life in five European countries. Clin Nutr 2010;29:726–732.
- Brown A: Differences in eating behaviour, well-being and personality between mothers following baby-led vs traditional weaning styles. Matern Child Nutr 2016;12:826–837.
- Brown A, Lee M: Maternal control of child feeding during the weaning period: differenc- es between mothers following a baby-led or standard weaning approach. Matern Child Health J 2011;15:1265–1271.
- Taylor RW, Williams SM, Fangupo LJ, et al: Effect of a baby-led approach to complemen- tary feeding on infant growth and over- weight: a randomized clinical trial. JAMA Pediatr 2017;171:838–846.
- Fangupo LJ, Heath AM, Williams SM, et al: A baby-led approach to eating solids and risk of choking. Pediatrics 2016;138:e20160772.
- Gooze RA, Anderson SE, Whitaker RC: Pro- longed bottle use and obesity at 5.5 years of age in US children. J Pediatr 2011;159:431– 436.
- Black MM, Hurley KM: Responsive feeding: strategies to promote healthy mealtime inter- actions; in Black RE, Makrides M, Ong KK (eds): Complementary Feeding: Building the Foundations for a Healthy Life. Nestlé Nutr Inst Workshop Ser. Vevey/Basel, Nestec/ Karger, 2017, vol 87, pp 153–165.
- Pan American Health Organization: Guiding Principles for Complementary Feeding of the Breastfed Child. Washington, Pan American Health Organization, World Health Organi- zation, 2003.
- Hohman EE, Paul IM, Birch LL, Savage JS: INSIGHT responsive parenting intervention is associated with healthier patterns of dietary exposures in infants. Obesity (Silver Spring) 2017;25:185–191.
- Daniels LA, Mallan KM, Nicholson JM, et al: An early feeding practices intervention for obesity prevention. Pediatrics 2015;136:e40– e49.