Causes of Stunting and Preventive Dietary Interventions in Pregnancy and Early Childhood
Abstract
Stunting of linear growth, a highly prevalent problem in children of low- and middle-in- come countries, is the result of the exposure of the fetus and/or young child to nutritional deficiencies and infectious diseases. Maternal undernutrition results in fetal growth restriction, and infectious diseases in pregnancy can result in preterm delivery. Both of these conditions are important contributors to stunting in early childhood, albeit their relative contribution varies by world region. After birth, growth faltering may begin at 3–5 months of life and becomes more prominent from 6 to 18 months. During this time, the young child is exposed to many infectious diseases, such as diarrhea, that have an adverse effect on growth. There is also increasing evidence that frequent ingestion of microorganisms results in damage to the small intestine. The resulting condition, referred to as environmental enteric dysfunction, even without clinical symptoms, may cause growth faltering. The complementary foods that the child receives in addition to breast milk are often inadequate in nutrients and energy, negatively affecting growth. Harmful exposure during pregnancy and the first 2 years of life, a critical period for growth and development, has led to a programmatic focus on this “1,000 days” in the life cycle. Dietary interventions, including nutrition education and for undernourished women provision of food supplements during pregnancy, result in improvements in fetal growth that position the newborn for healthier growth. Interventions in the first 2 years of life include promotion of exclusive breastfeeding for the first 6 months of life and continued breastfeeding for at least the first 2 years, nutritional counseling to assure adequate complementary feeding, and, if necessary in food insecure areas, the provision of supplemental food to be given to the child. Evidence shows that each of the interventions has a beneficial effect on the growth of the young child, yet that the effect is modest in relation to the degree of stunting observed in these underprivileged populations. Nevertheless, in recent years, reductions in the prevalence of stunting in some low-income countries show that substantial improvements are possible as a result of socioeconomic changes along with specific infection control and dietary interventions.
Introduction
Stunting of growth in length in early childhood has been associated with in- creased mortality and morbidity, reduced cognitive ability, adult metabolic dis- eases, and reduced earning potential [1, 2]. Globally, in 2015, 156 million children had stunted growth, or 23% of all children under 5 years of age [3]. The broad consequences and high prevalence of stunting in low- and middle-income countries (LMIC) resulting in a high burden of disease make it a high priority for public health interventions [4].
There are numerous causes of stunting [1]. At a distal level, social, economic, and environmental factors, including poor governance, misguided policies and politics, weak leadership, and limited technical capacity in nutrition, are important determinants. At an intermediate level, contributing factors include food insecurity, insufficient caregiving resources, unsafe and unhygienic housing conditions, and limited access and utilization of health services. At this level, nutrition-sensitive programs and approaches for agriculture and food security, social safety nets, women’s empowerment, child protection, availability of high- quality health services, and water/sanitation could beneficially affect growth and development in childhood. At a proximal level, providing nutrient-rich foods, ensuring good feeding and caregiving practices, and controlling exposure to infectious agents are critical to support healthy growth. It is at this level that this chapter will consider the intergenerational causes of stunting and the dietary interventions in pregnancy and early childhood to prevent it from occurring.
Prenatal Origins of Stunting and Maternal Nutritional Interventions
Fetal growth restriction and preterm delivery are important risk factors for the development of stunting in young children [1]. An analysis of birth cohort data from 19 studies in LMIC compared the risk of subsequent stunting for babies with these conditions versus those born appropriate for gestational age (AGA) at term [5]. Babies born preterm and AGA, at term and small for gestational age (SGA), and both preterm and SGA had odd ratios (OR) for stunting of 1.93 (95% confidence interval, CI, 1.71–2.18), 2.43 (95% CI 2.22–2.66), and 4.51 (95% CI 3.42–5.93), respectively. Taking the increased risk and the prevalence of these birth outcomes in world regions into account, the population-attributable risk for childhood stunting was 4% for preterm AGA, 16% for term SGA, and 4% for preterm SGA. Thus, about a quarter of childhood stunting in LMIC may have origins prior to delivery.
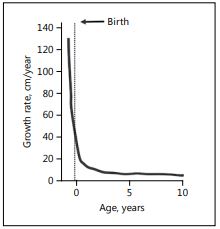
Fig. 1. Growth rate from conception to age 10 years.
The growth rate (Fig. 1) of the fetus is very high (>100 cm/year) early in gestation declining to about 50 cm/year at birth [6]. There are many conditions that can adversely affect fetal growth, including maternal infections and other morbidities, which may also increase the risk of premature delivery [1]. This discussion will focus on the demographic factors and nutritional conditions that are associated with fetal growth restriction and being SGA at birth. In an analysis of 14 cohort studies in LMIC, nulliparous women had a higher risk of having an SGA baby in the groups <18 and 18–34 years: adjusted OR 1.80 (95% CI 1.62–2.01) and 1.51 (95% CI 1.39–1.64), respectively [7]. For older-age mothers (≥35 years), there was no increase in the risk of SGA. Birth intervals of <18 months significantly increased the risk of SGA: adjusted OR 1.51 (95% CI 1.31–1.75) [8]. Possible biological explanations for the demographic associations are that young mothers may have incomplete physical growth and more undernutrition [9], and that women with short spacing between pregnancies may have maternal nutritional depletion, although the evidence for the latter is mixed [10].
Maternal stature reflects genetic and environmental factors but is itself strongly related to the growing periods in early childhood, and adult short stature is in part a consequence of childhood stunting. An important trial in Guatemala demonstrated that nutritional supplementation in early child- hood had beneficial nutritional effects not only on the study participants but also on the second-generation offspring for children in the supplementation group, who had higher birth weights and greater head circumferences [11].
Short stature is significantly associated with SGA; in an analysis of 12 cohort studies, women <145 cm had an adjusted OR of 2.03 (95% CI 1.76–2.35) for term SGA [12]. Delay of pregnancy in adolescents would allow for a longer time for growth in late puberty, but maternal stature cannot be changed at the time of pregnancy. This demonstrates the intergenerational aspects of stunting and the need to focus on preventing fetal growth restriction and growth faltering in early childhood that leads to maternal short stature. A number of other nutritional factors, including micronutrient deficiencies, which are considered in the chapter by Sharma et al. [this volume, pp. 115–126], moderate-to-severe anemia [13], weight gain during pregnancy, and maternal body mass index (BMI), are associated with the risk of SGA births. In an analysis of 8 cohorts, women with low BMI (<18.5) had an increased risk of SGA (relative risk 1.41 [1.24– 1.60]) [1].
Nutritional interventions in pregnancy have ranged from dietary advice to provision of balanced energy protein supplements, providing about 25% of the total energy as protein, which is recommended for undernourished pregnant women. A systematic review concluded that balanced energy protein supplementation results in a reduction of 34% in the risk of SGA births, with greater effects in less-well-nourished women [14].
Nutritional Causes of Stunting in Early Childhood and Dietary Interventions
Current recommendations are that newborns should be put to the breast within 1 h of birth and be exclusively breastfed for the first 6 months, and breastfeeding should be continued until at least 2 years of age [15]. There is an increased risk of infectious diseases and mortality with deviation from these recommendations, especially in the first 6 months when introduction of contaminated food and water leads to greater exposure to enteric pathogens [1, 15]. It has been commonly thought that such deviations will result in growth faltering as a consequence of both infections and introduction of food of poorer quality than breast milk. Some observational studies have found an association of poorer breast- feeding practices with linear growth in infancy [16]. However, more carefully controlled studies, including randomized trials, have not found that promotion of breastfeeding resulting in practices closer to the recommendations has an effect on linear growth [15].
Complementary feeding includes the provision of nutritionally rich foods in addition to breast milk from about 6 months of age and continuing until breastfeeding ceases. Observational studies have found associations between dietary factors and stunting. Using survey data from 7 Latin American countries, there was a significant association between complementary feeding practices and height-for-age z-scores [17]. In community-based studies in Indonesia, there was a positive effect on growth with an increased number of complementary feeds per day [18], and in Bangladesh there was a benefit of high dietary diversity [19]. Data from the Demographic and Health Surveys (DHS) from 11 countries were used to create a dietary diversity score that was positively associated with linear growth in 9 of the countries [20]. Additional work using DHS data showed that consumption of a minimum acceptable diet with dietary diversity reduced the risk of stunting [21]. These indicators of infant and young child feeding have been used extensively at population level to understand determinants of poor growth and to monitor dietary intervention programs, but more specific and refined measures are needed for analyses at the individual level [22]. Dietary interventions include caregiver education or counseling about appropriate complementary feeding practices or the provision of supplemental foods [23]. Nutrition education may emphasize the importance of continuing breastfeeding, offering diverse nutrient-dense foods, and providing feeding at a frequency appropriate for the age and type of complementary foods (less-nutrient-dense foods need to be fed more frequently). Nutrition education interventions are most appropriately implemented in food-secure settings where recommended dietary changes should be possible. In food-insecure settings, food supplements of various types, along with nutrition education, are often used to enhance complementary feeding. A recent systematic review considered randomized controlled trials and controlled before/after studies in which children 6–23 months of age were targeted for a complementary feeding intervention for at least 6 months [24]. Nine studies of nutrition education and 8 studies of nutritional supplementation contributed to the analysis. The nutrition education interventions had a statistically significant effect of small size, i.e., mean difference in length-for-age z-score (LAZ) of 0.22 (95% CI 0.08–0.37) compared to the control groups (Table 1). Nutrition education in food-insecure populations had no effect on LAZ. In food-insecure settings, nutritional supplementation interventions combining the results of 7 studies showed a statistically significant but small benefit compared to controls, with a difference in LAZ (using the WHO growth standard for calculation) of 0.10 (95% CI 0.03–0.17). There were insufficient studies of nutrition education alone in food-insecure settings, reflecting the belief that enhanced complementary feeding recommendations could not be implemented if families lack resources and available foods.
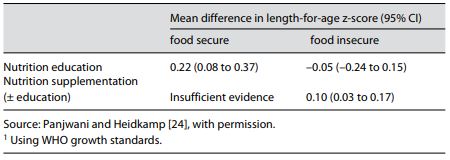
Table 1. Effect of interventions on length-for-age z-score1 in children 6–23 months old in food-secure and -insecure settings
Infections in Childhood Contribute to Stunting
The contribution of infectious diseases to the development of stunting in early childhood has been recognized for many decades [25]. With the introduction of food and water, which are often contaminated in LMIC, to the child’s diet, the incidence of infectious diarrhea is very high in the first 2 years of life: commonly 4 or more episodes of illness occur per year [26]. In an analysis of cohort data from multiple LMIC, 25% of stunting in children at 2 years of age could be attributed to the child having 5 episodes of diarrhea before that age [27]. The effect of diarrhea on growth faltering and stunting in this age group will depend on the adequacy of the diet, appropriate treatment of the illness, including continuation of feeding, and the length of the convalescence period, which may permit recovery from growth faltering during illness. Associations of other common childhood illnesses, such as acute respiratory infections or other febrile illnesses, with growth faltering have been found but are not as consistent as for diarrhea [28].
There is also increasing evidence that exposure of the intestine to enteric pathogens and other microbes can result in flattened intestinal villi, mucosal inflammation, reduced barrier integrity, and malabsorption of nutrients. This condition, termed environmental enteric dysfunction, is hypothesized to adversely affect growth [29]. In addition to these subclinical changes in the gut, activation of the systemic immune system and inflammation from repeated exposures to microorganisms may have metabolic costs and result in changes in vitamins and minerals in the body that may result in growth faltering. Control of exposure of the young child to contaminated food, water, and the environment may be very important for a reduction in the prevalence of stunting.
Conclusions
Growth in young children has important determinants from conception to the first 2 years of life, referred to as the 1,000 days [1]. This critical period for growth and the influence of adverse effects that lead to stunting has attracted increased attention in the last decade after calls for greater focus of nutritional programs in LMIC [4]. The increasing appreciation that this period is also critical for brain development and cognitive abilities has added to the priority for effective interventions in these segments of the life cycle [30].
Interventions providing nutrition education in food-secure settings and nutritional supplementation in food-insecure settings have detectable benefits on linear growth. However, the size of these effects, just a small fraction of an LAZ improvement when the length deficit is 1.5–2.0 z-scores by age 2 years [31], shows that we are not yet able to make much difference in the high prevalence of stunting in LMIC. Improvements in social determinants have been found to be important correlates of a decline in the prevalence of stunting in cross-country [32] and national analyses [33]. Income growth, women’s empowerment, and social safety nets, e.g., cash transfers, and nutrition-sensitive agriculture programs are likely supportive of better child growth, although better evidence is needed on how important they are and how to maximize benefits in different settings [32]. It is also likely that frequent infectious diseases and exposure to enteric microbes contribute to growth faltering and must be addressed at the same time as dietary interventions are implemented [28]. Continued reductions in the prevalence of stunting with social and economic improvements are anticipated, and more research is needed to learn how to accelerate the reductions with dietary interventions.
Disclosure Statement
R.E. Black is a member of the Creating Shared Value Advisory Council of the Nestle Co. and is on the governing boards of Nutrition International and Vitamin Angels. R. Heidkamp has no disclosures.
References
- Black RE, Victora CG, Walker SP, et al: Maternal and child undernutrition and over-weight in low-income and middle-income countries. Lancet 2013;382:427–451.
- Victora CG, Adair L, Fall C, et al: Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008; 371:340–357.
- UNICEF, World Health Organization, The World Bank: Global Database on Child Growth and Malnutrition. Joint Child Malnutrition Estimates – Levels and Trends. 2016, http://www.who.int/nutgrowthdb/en/.
- The Maternal Child Nutrition Study Group: Black RE, Alderman H, Bhutta Z, et al: Maternal and child nutrition: building momentum for impact. Lancet 2013;382:372–375.
- Christian P, Lee SE, Donahue Angel M, et al: Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int J Epidemiol 2013;42:1340–1355.
- Lui JC, Baron J: Mechanisms limiting body growth in mammals. Endocr Rev 2011;32: 422–440.
- Kozuki N, Lee AC, Silveira MF, et al: The associations of parity and maternal age with small-for-gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Public Health 2013;13(suppl 3):S2.
- Kozuki N, Lee AC, Silveira MF, et al: The as- sociations of birth intervals with small-for- gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Public Health 2013;13(suppl 3):S3.
- Gibbs CM, Wendt A, Peters S, Hogue CJ: The impact of early age at first childbirth on ma- ternal and infant health. Paediatr Perinat Epi- demiol 2012;26(suppl 1):259–284.
- Conde-Agudelo A, Rosas-Bermudez A, Cas- tano F, et al: Effects of birth spacing on maternal, perinatal, infant, and child health: a systematic review of causal mechanisms. Stud Fam Plann 2012;43:93–114.
- Martorell R, Zongrone A: Intergenerational influences on child growth and undernutrition. Paediatr Perinat Epidemiol 2012; 26(suppl 1):302–314.
- Kozuki N, Katz J, Lee AC, et al: Short mater- nal stature increases risk of small-for-gestational-age and preterm births in low- and middle-income countries: individual participant data meta-analysis and population attributable fraction. J Nutr 2015;145:2542– 2550.
- Kozuki N, Lee AC, Katz J, et al: Moderate to severe, but not mild, maternal anemia is associated with increased risk of small-for-gestational-age outcomes. J Nutr 2012;142:358– 362.
- Imdad A, Bhutta ZA: Maternal nutrition and birth outcomes: effect of balanced protein- energy supplementation. Paediatr Perinat Epidemiol 2012;26:178–190.
- Victora CG, Bahl R, Barros AJ, et al: Breast- feeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016; 387:475–490.
- Adair LS, Guilkey DK: Age-specific determinants of stunting in Filipino children. J Nutr 1997;127:314–320.
- Ruel MT, Menon P: Child feeding practices are associated with child nutritional status in Latin America: innovative uses of the Demo- graphic and Health Surveys. J Nutr 2002;132: 1180–1187.
- Schmidt MK, Muslimatun S, West CE, et al: Nutritional status and linear growth of Indonesian infants in West Java are determined more by prenatal environment than by postnatal factors. J Nutr 2002;132:2202–2207.
- Rah JH, Akhter N, Semba RD, et al: Low di- etary diversity is a predictor of child stunting in rural Bangladesh. Eur J Clin Nutr 2010;64: 1393–1398.
- Arimond M, Ruel MT: Dietary diversity is associated with child nutritional status: evi- dence from 11 demographic and health sur- veys. J Nutr 2004;134:2579–2585.
- Marriott BP, White A, Hadden L, et al: World Health Organization (WHO) infant and young child feeding indicators: associations with growth measures in 14 low-income countries. Matern Child Nutr 2012;8:354– 370.
- Ruel MT: Measuring infant and young child complementary feeding practices: indicators, current practice, and research gaps; in Black RE, Makrides M, Ong KK (eds): Complementary Feeding: Building the Foundations for a Healthy Life. Nestlé Nutr Inst Workshop Ser. Vevey/Basel, Nestec/ Karger, 2017, vol 87, pp 73–87.
- Bhutta ZA, Das JK, Rizvi A, et al: Evidence- based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet 2013;382:452–477.
- Panjwani A, Heidkamp R: Complementary feeding interventions have a small but significant impact on linear and ponderal growth of children in low- and middle-income countries: a systematic review and meta-analysis. J Nutr 2017;147:2169S–2178S.
- Scrimshaw NS, Taylor CE, Gordon JE: Inter- actions of nutrition and infection. Monogr Ser World Health Organ 1968;57:3–329.
- Walker CL, Rudan I, Liu L, et al: Global bur- den of childhood pneumonia and diarrhoea. Lancet 2013;381:1405–1416.
- Checkley W, Buckley G, Gilman RH, et al: Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol 2008;37:816–830.
- Black RE: Patterns of growth in early child- hood and infectious disease and nutritional determinants; in Black RE, Makrides M, Ong KK (eds): Complementary Feeding: Building the Foundations for a Healthy Life. Nestlé Nutr Inst Workshop Ser. Vevey/Basel, Nest- ec/ Karger, 2017, vol 87, pp 63–72.
- Keusch GT, Denno DM, Black RE, et al: Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clin Infect Dis 2014;59(suppl 4):S207–S212.
- Black MM, Walker SP, Fernald LC, et al: Early childhood development coming of age: sci- ence through the life course. Lancet 2017;389: 77–90.
- Victora CG, de Onis M, Hallal PC, et al: Worldwide timing of growth faltering: revis- iting implications for interventions. Pediatrics 2010;125:e473–e480.
- Ruel MT, Alderman H, The Maternal Child Nutrition Study Group: Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving ma- ternal and child nutrition? Lancet 2013;382: 536–551.
- Huicho L, Huayanay-Espinoza CA, Herrera- Perez E, et al: Factors behind the success sto- ry of under-five stunting in Peru: a district ecological multilevel analysis. BMC Pediatr 2017;17:29