Controversies on the role of hydrolysed formulas in the prevention of allergy and management of cow’s milk protein allergy in infants
There are controversies surrounding the role of hydrolysed formulas (HF) in the prevention of allergy and management of cow’s milk protein allergy (CMPA) in infants.
Controversies on the role of hydrolysed formulas in the prevention of allergy and management of cow’s milk protein allergy in infants
This satellite symposium took place on 27th October 2017, as part of the Pediatric Allergy and Asthma Meeting (PAAM) 2017 in London, UK, a focussed meeting from the European Academy of Allergy and Clinical Immunology (EAACI).
Chairperson: Antonella Muraro1 . Speakers: Hania Szajewska,2 Kirsi Laitinen,3 Martinas Kuslys4
1. The Referral Centre for Food Allergy Diagnosis and Treatment, Veneto Region, Department of Mother and Child Health, University of Padua, Padua, Italy. 2. The Medical University of Warsaw, Department of Paediatrics, Warsaw, Poland. 3. Institute of Biomedicine, Faculty of Medicine, University of Turku, Turku, Finland. 4. Pediatric Care Nestlé Health Science, Lausanne, Switzerland.
MEETING SUMMARY
The satellite symposium involved a discussion on the controversies surrounding the role of hydrolysed formulas (HF) in the prevention of allergy and management of cow’s milk protein allergy (CMPA) in infants. The aims were to understand the importance of the selection criteria of studies in meta-analyses and to gain an appreciation of the specific considerations when interpreting meta-analyses; to discuss safe and suitable nutritional management for infants with CMPA; and to explore the heterogeneity of the hydrolysates used in extensively HF (eHF) intended for the management of infants with CMPA, and the need for a total quality management approach.
Professor Szajewska opened the symposium by overviewing the importance of meta-analyses, explaining how different conclusions may be reached based on interpretation, and summarising the evidence for the role of HF in allergy prevention and the recommendations from various guidelines. Adjunct Professor Laitinen then discussed the importance of making appropriate choices in the dietary management of infants with CMPA, particularly during different stages of development. The benefits of breastfeeding and key factors to consider when selecting infant formula were outlined. Dr. Kuslys closed the session by considering the question: ‘Is extensive hydrolysis enough to guarantee success?’. Data from an analytical programme aiming to improve the understanding of the wide range of commercially available eHF using a range of techniques were presented, with an emphasis on the need for multiple analytical methods to fully characterise eHF.
Hydrolysed Formula for Allergy Prevention: The Role of Meta-Analyses
Professor Hania Szajewska
Medical University of Warsaw, Poland
The session began with a focus on the importance of metaanalyses and systematic reviews in evaluating the effectiveness of any given intervention. Although only a general guideline, the hierarchy of evidence1 provides a useful overview of the various evidence types, with systematic reviews and meta-analyses considered to offer the strongest evidence followed by (in descending order of evidence strength) randomised controlled trials (RCT), cohort studies, case-control studies, case series studies, and lastly, expert opinion or theories, basic research, and animal studies.
Systematic reviews involve the systematic identification, collection, and analysis of data to answer a well-formulated clinical question, and ultimately, each RCT is discussed separately. A meta-analysis begins with a systematic review; however, statistical techniques are then used to pool the results of all the RCT so that they can be analysed together. The primary reasons for performing a meta-analysis are to increase statistical power, thus increasing the chance to reliably detect a clinically important difference and to improve precision in estimating effects, enabling the confidence interval around the effects to be narrowed.2 When determining whether results of individual trials should be pooled or kept separate, it is important to consider if the studies are sufficiently homogeneous in terms of both the question and the methods. PICO serves as a mnemonic for the four factors that should be considered and should ideally be the same across studies to be pooled: population, intervention, comparison, and outcome.2
It is a rare occurrence for different studies to be truly homogenous, particularly HF intervention studies, which can vary greatly. A critical reason for this is the arbitrary distinction between partially hydrolysed formulas (HF) (antigenreduced) and extensively HF (eHF) (almost antigen-free); currently, there is no consensus on the criteria to distinguish between the two.3
A recent systematic review and meta-analysis by Boyle et al.4 considered HF and the risk of allergic or autoimmune disease. The conclusion of the meta-analysis stated that there was “no consistent evidence to support the use of HF for the prevention of allergic disease.” These findings generated much discussion and were deemed controversial, reasons for which included the fact that data from various types of partially and eHF were pooled together, and that various study designs (i.e., randomised and non-randomised trials) were also pooled together. In addition, studies applying additional interventions, such as house dust mite control measures and a smoke-free environment, were included in one group but not the other. The reviewers also selected time intervals for the age of assessment of 0-4, 5-14, and >15 years, therefore making a subjective decision as to which data should be included.
The great variation among HF was emphasised, with not all HF providing the same degree of protective benefit,5 and numerous factors, such as protein source, hydrolysis method, and degree of hydrolysis, are manufacturer-dependent and contribute to differences between HF. Professor Szajewska highlighted that the efficacy and safety should therefore be established for each HF.
Another meta-analysis6 that considered intervention with one partially hydrolysed 100% whey formula and the risk of eczema and allergy prevention was presented. Methodology developed by the Cochrane Collaboration was used, with 13 publications identified, reporting on 8 RCT. Figure 1 displays the methodological qualities of the trials included in the meta-analysis. Compared with the Boyle et al.4 meta-analysis, the key differences were that only one HF was included (a partially hydrolysed 100% whey-based formula), and that only time intervals reported by the authors in the original publications were used.
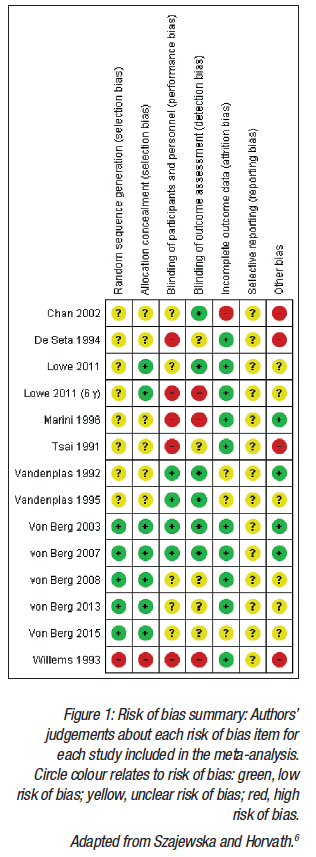
For eczema, the intention-to-treat analysis included exclusively breastfed infants, including those who were never exposed to the partially HF. The pooled analysis demonstrated a reduced risk of eczema, which was statically significant for some but not all of the time points (Figure 2). The per-protocol analysis only included participants who adequately adhered to the assigned regimen. Similar to the intention-to-treat analysis, results from the per-protocol analysis showed a reduced risk of eczema, which was statically significant for some but not all of the timepoints.6 It was noted that while per-protocol analyses usually best reflect the effects of treatment in everyday practice, attrition bias can be introduced, whereby the groups of patients being compared no longer have similar characteristics.
Interestingly, the two studies that contributed most to the pooled results, the Melbourne Atopic Cohort Study (MACS)7 and the German Infant Nutritional Intervention (GINI) study,8-12 showed opposite findings. MACS, a single-blind RCT, concluded that there was no evidence that the introduction of partially hydrolysed whey-based formula and the cessation of breastfeeding reduced the risk of allergic manifestations in high-risk infants. Conversely, the GINI study, a double-blind RCT, demonstrated that the allergy preventative effect of extensively hydrolysed casein formula and partially hydrolysed whey formula appeared in the first year of life and persisted until the age of 6 years. Effects on the prevalence of some allergic and respiratory symptoms were also demonstrated at 15 years after early intervention. A fundamental difference between the MACS and GINI studies was the methods used to assess outcomes. In the MACS study, any allergic manifestations were assessed during telephone interviews, whereas in the GINI study, a diagnosis of atopic dermatitis had to be confirmed by a second physician.
The question of whether allergy prevention with HF is cost-effective was considered. Results from three separate studies conducted in five European countries,13 in Germany,14 and in Australia,15 all confirmed that the use of a HF would be a cost-effective, or even cost-saving, measure. Until recently, the recommendations in various guidelines for HF in allergy prevention consistently endorsed the use of a hypoallergenic formula with a documented preventive effect (if the infant was not breastfed).5,16-19 However, different opinions stemming from the Boyle et al.4 meta-analysis now exist, which translate into differences in the recommendations, with some citing that inconsistencies or methodological flaws in the studies limit the evidence to support recommendations.
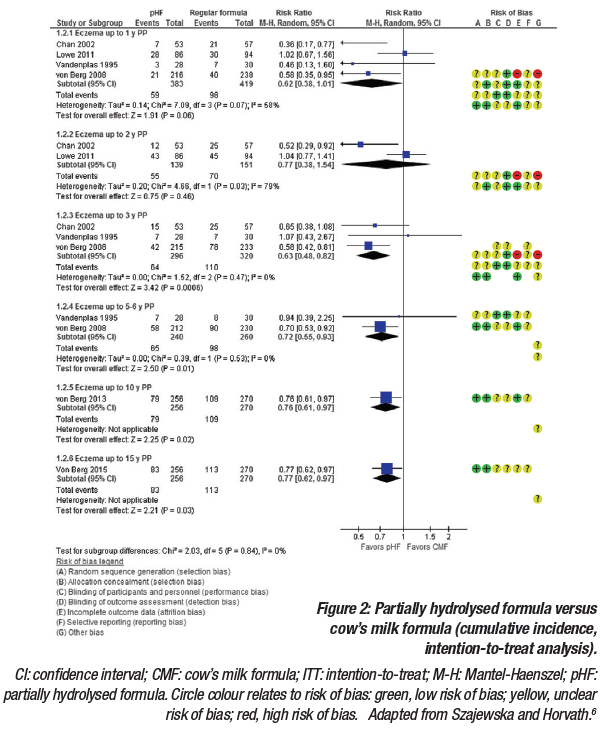
In summary, meta-analyses can reach different conclusions for numerous reasons and there are specific considerations when interpreting the findings of a meta-analysis evaluating the role of HF. The design of included studies must be considered, and if non-RCT were included alongside RCT, they should be evaluated separately. If participants were included from both high-risk and general populations, they should also be evaluated separately. In terms of outcomes, it is important to confirm exactly how the allergic disease diagnosis was made; preferentially, diagnosis should be based on widely agreed criteria and confirmed by a physician. Perhaps most importantly, HF should not be considered equal, and therefore efficacy should be evaluated separately.
Dietary Management of Cow’s Milk Protein Allergy in Infants: Ensuring Safety and Suitability
Adjunct Professor Kirsi Laitinen
University of Turku, Finland
The vast importance of making appropriate choices in the dietary management of infants with cow’s milk protein allergy (CMPA) was discussed. By managing CMPA with an appropriate elimination diet, the effects on the infant can be multifaceted; gut epithelial function is normalised and immune function modified, resulting in remission of symptoms and enabling growth and development to reach genetic potential. There are also effects on the intestinal microbiota and potential implications on long-term health. The benefits of diverse complementary feeding were also highlighted, as fats and fibres also have immunomodulatory properties and influence the intestinal microbiota.
The numerous causes for the deterioration of nutritional status and growth failure in children were overviewed. Primarily, these stem from an inadequate dietary intake, increased nutrient losses from the gut, and increased energy and nutrient requirements of the child.20 Low intake or poor use of nutrients can arise from a poor appetite and gut symptoms, which can be a result of a restricted diet due to elimination, poor dietary guidance, and acquired dietary habits such as refusal of, or aversion to, certain foods. Children may also have enhanced immunoinflammatory responses or an impaired intestinal barrier function, which can contribute to gut symptoms, such as diarrhoea, and affect the bioavailability of food and nutrients.21 Increased dietary requirements can also be impacted by persistent inflammation23 and agitation.24 Growth is a good indicator of nutritional status and can be negatively affected by severe atopic disease,25-27 multiple allergies and food avoidances,28-32 and early onset of disease.33 In one study it was observed that when children with CMPA are diagnosed, they often exhibit poor growth compared with normal reference values; however, when fed an eHF for 6 months, Scoring Atopic Dermatitis (SCORAD) scores, weight-forage, length-for-age, and weight-for-length z-scores all improved.34
Breastfeeding is known to have numerous benefits, such as providing protection from infections and obesity, promoting neural and visual development and mother-child bonding, and a positive effect on maternal recovery following delivery. The complex composition of breastmilk includes nutrients, antiinflammatory and antimicrobial factors, growth factors, hormones, enzymes, and prebiotic factors, and varies during each feed, throughout the feeding period, and according to the mother’s diet. Whilst some fragments of cow’s milk protein, such as ß-lactoglobulin, exist in breastmilk, the concentrations of these are minimal compared with cow’s milk. Thus, it is advised that children with CMPA should be breastfed (provided there is no adverse reaction), which, along with nutritional benefits, may also induce oral tolerance.
There are numerous decisions to be made when feeding children with CMPA, such as choosing the most suitable formula in cases where the infant cannot be breastfed, and making age-appropriate choices at different stages of development. Speciality infant formulas for CMPA can be categorised according to the source of the protein, for example, amino acid-based (non-allergenic) formulas, extensively hydrolysed whey or casein-based formulas (derived from cow’s milk), rice-based formulas, and soya-based formulas that can contain intact or hydrolysed protein. Milk from other mammals should not be used for the dietary management of CMPA due to cross-reactivity, and neither should alternative milks (e.g., soya, oat, or rice) because they are not designed for use in infants. In addition to protein, it is also important to consider the overall nutritional composition of a specialty formula.
Extensively hydrolysed formulas (eHF) are regarded as the first choice, according to treatment guidelines, and are suitable for most infants with CMPA. Amino acid-based formulas are the next option if eHF are unsuitable and are reserved for children with the most severe disease (i.e., those presenting with faltering growth, eosinophilic oesophagitis, multiple food allergies, and/or a high risk of anaphylaxis); however, amino acid-based formulas are associated with problems due to taste and high cost. Newer formulas, such as those based on rice, have less supporting evidence; however, they have been used for >10 years in some countries.35 Rice-based formulas have the benefits of rarely inducing allergenicity and are relatively low cost. Soya-based formulas have a good composition of amino acids and taste, and are also low cost; however, concerns exist over children developing soya allergies and the potential to generate hormonal responses. Although there is little evidence to support this, soya-based formulas are only recommended for infants older than 6 months as a precaution.
The approach to feeding infants with CMPA varies depending on age. Whilst breastmilk should be given wherever possible, supplementing with infant or follow-on formula is often necessary. In children with CMPA, the use of formulas may continue after the first year, up to 2 years, and in some cases even longer if required. Children with CMPA are recommended to start eating solid foods at the same age as those without CMPA (~4-6 months), with guidance recommended for parents to understand labelling on packaging and avoid cross-contamination of foods.
There are several recognised challenges to maintaining nutritional adequacy in children with CMPA. A delay in making the correct diagnosis can contribute to these challenges, as can a restricted diet due to multiple allergies, uncoordinated elimination, poor guidance, feeding difficulties, and acquired dietary habits such as food aversion. Risk of anaphylaxis and fear of contamination can also lead to further dietary restrictions. In some studies,29,36-42 these factors have resulted in a low dietary intake of energy and nutrients (such as protein, zinc, and calcium). However, the use of hypoallergenic formula may be particularly beneficial in ensuring sufficient dietary intake.31
These complex challenges highlight the importance of ensuring appropriate dietary management and the pivotal role of a dietician in individual counselling and in the training of other healthcare professionals. The main aims of dietary management in CMPA are to support normal growth and development, obtain relief of symptoms by avoidance of allergenic foods, and prevent inadvertent exposure to allergenic foods (whilst preventing unnecessary avoidance of foods), along with ensuring an adequate, healthy, and balanced diet with appropriate alternatives for excluded food allergens to minimise the impact on quality of life.43
Extensively Hydrolysed Formulas for the Management of Cow’s Milk Protein Allergy in Infants: Is Extensive Hydrolysis Sufficient to Guarantee Success?
Doctor Martinas Kuslys
Nestlé Health Science, Switzerland
There are currently no aligned, actionable definitions to characterise extensively hydrolysed formulas (eHF), which is reflected in the wide variation of the degree of hydrolysis of commercially available eHF. This is despite the fact that all eHF are intended for the same purpose: to be well-tolerated by most infants with CMPA and to be nutritionally complete with a similar taste and consumption properties to regular formulas.
Both chemical analysis5,44 and desired clinical outcome45-47 can be used to characterise eHF and recent publications have drawn attention to the chemical heterogeneity of eHF. The peptide profiles of three whey-based eHF were analysed and each were found to be different;48 two contained residual whey peptides, recognised by specific immunoglobulin E, and two had residual caseins, with the authors concluding that “the degree of hydrolysis and the size of residual peptides of each eHF should be known by practitioners.” Lambers et al.49 tested four peptide profiles from four batches of several commercially available casein-based eHF, and, similarly, found each to be different. The authors speculated that variations in peptide profiles may be linked to differences in overall functionality. Interestingly, few eHF products have been proven in clinical trials to be efficient in terms of both allergy and growth.45
Samples of commercially available eHF from 11 countries and various manufacturers were analysed with a clear focus on suitability for CMPA management to better understand the range of eHF. Only eHF marketed for the management of CMPA in infants and based on milk proteins were included. Both internal investigations at the Nestlé Research Labs, Lausanne, Switzerland, and external investigations at Neotron SpA, Modena, Italy, contributed to the programme. Samples were de-identified and coded, and all research was conducted in accordance with international testing standards.
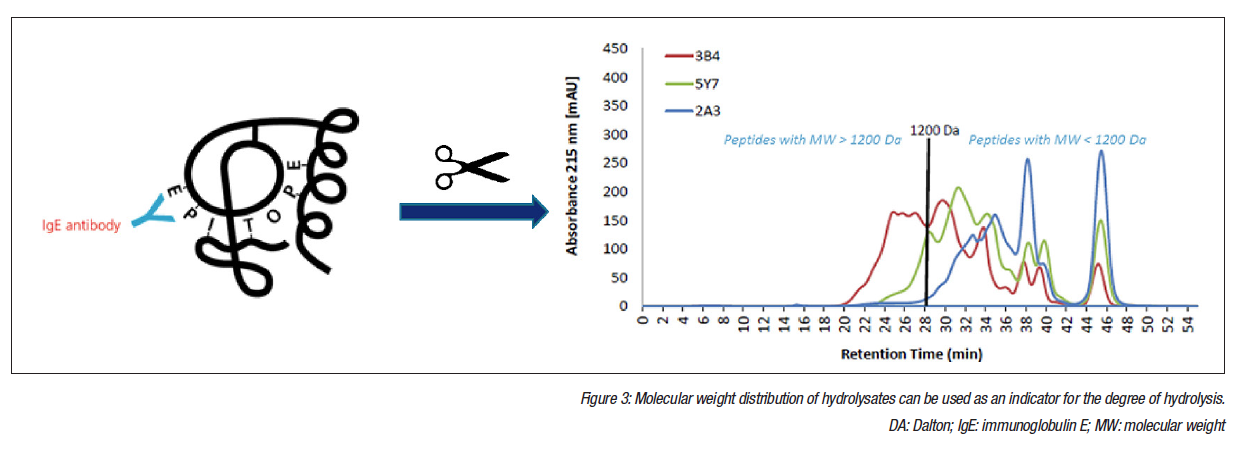
Whilst molecular weight (MW) distribution of hydrolysates is a good indicator of the degree of hydrolysis, additional parameters should be used to define eHF (Figure 3). Quantifying residual proteins provides valuable information because these data reflect both the design of the formula and the quality management during production. Osmolarity, nitrogen fractions, lactose content, total and free amino acids, ß-lactoglobulin, and casein content were included in the analysis, and sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and size-exclusion high-performance liquid chromatography (SEHPLC) were used for peptide profiling and MW distribution analysis. Results concentrated on peptide profiles and MW distribution, and ß-lactoglobulin and casein content were determined by enzyme-linked immunosorbent assay (ELISA).
Surprisingly, peptide MW distribution displayed significant variation, with the percentage of peptides with a MW >1.2 kDa varying from 1-36%. Preliminary and ongoing work has investigated the link between MW distribution and allergenicity. MW distribution was compared with ß-lactoglobulin content in an in vitro degranulation assay to quantify the degranulation of basophils to concentrations of ß-lactoglobulin. Any results above the limit of detection were interpreted as residual allergenicity.50,51 In the preliminary experiments, six brands were selected, two from each of the following categories based on MW distribution: <3%, 3-15%, and >15% content of peptides with MW >1,200 Da. The results suggested a correlation between the concentration of larger peptides and degranulation, with the two brands that had the lowest quantity of larger peptides producing results below the detection limits of the degranulation test. Further research is planned to confirm these initial findings.
Interestingly, ß-lactoglobulin levels displayed variation of >2 orders of magnitude. In total, 20% of samples had a non-measurable ß-lactoglobulin content (smaller than or at the limit of quantification: 0.010 mg/kg); however, 80% of samples had a ß-lactoglobulin content greater than the limit of quantification, with high variability from 0.020-36.000 mg/kg. Figure 4 (overleaf) displays these data, which underline the importance of employing a combination of complementary analytical methods when assessing formulas. Surprisingly, even in the groups of samples that featured a high degree of hydrolysis, there were significant levels of residual ß-lactoglobulin. A potential explanation could be the company-specific production processes or contamination.
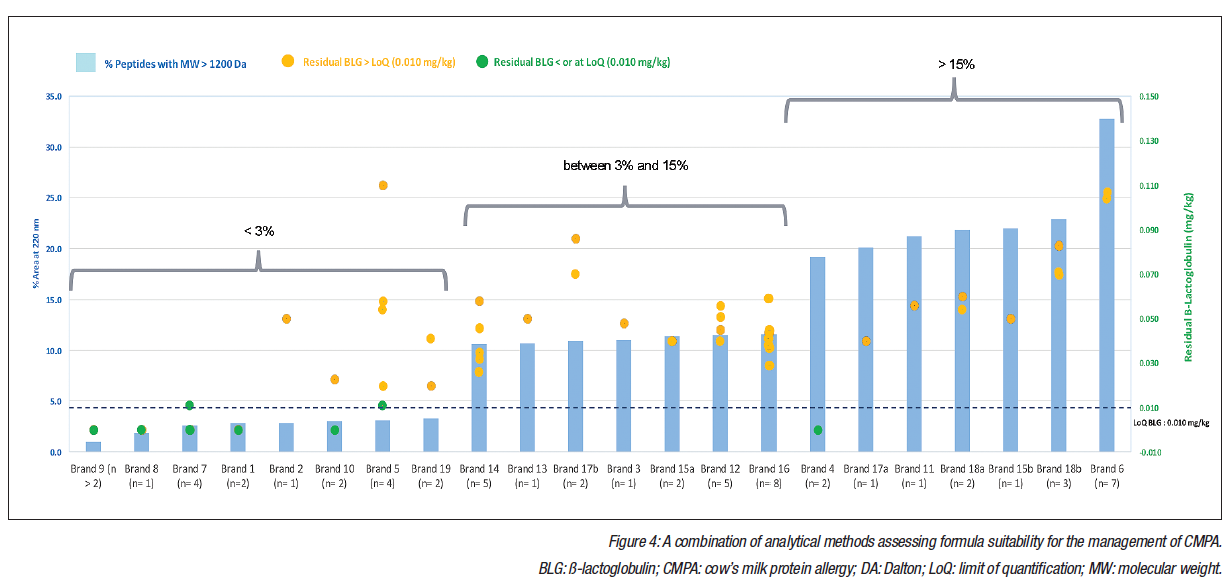
The European Academy of Allergy and Clinical Immunology (EAACI) has recognised that not all eHF are clinically tested or fit for their intended purpose.52 A lack of consensus over the definition of extensively hydrolysed is reflected in the wide range of the degree of hydrolysis in commercially available eHF, and can result in products that are mislabelled as extensively hydrolysed and may be high-risk or unsuitable for CMPA management. Results of these analyses of samples from 11 countries highlight that the degree of hydrolysis alone is not sensitive enough to characterise eHF, and that whilst a high degree of hydrolysis is desirable, further quality control measures are essential to ensure clinically safe products. Actionable guidelines should be implemented to improve how eHF are defined and to provide guidance on conducting clinical trials.
If you liked this post you may also like
