Role of Diet in Inflammatory Bowel Disease
- The number of new inflammatory bowel disease (IBD) patients is steadily increasing, especially in countries with a Western lifestyle.
- There is an evident link between the change of food habits/food production and the incidence of IBD.
- Experimental studies indicate that commonly used food ingredients can alter the intestinal barrier, thereby causing intestinal inflammation.
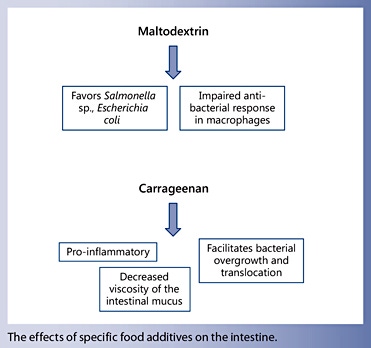
- Complex carbohydrates, such as maltodextrin, emulsifying agents or thickeners, such as carboxymethyl cellulose, carrageenan and xanthan gum were shown to have detrimental effects on intestinal homeostasis.
- Based on epidemiological and experimental studies, exclusion or defined diets were proposed.
- There is good evidence that exclusive enteral nutrition is a potent therapy to induce remission in patients with Crohn’s disease.
- For exclusion and defined diets to treat IBD, scientific proof is still lacking, but interesting studies are underway.
Major advances have been achieved over the last 15 years in the understanding of the pathophysiology of IBD. There is clear evidence that in genetically determined individuals (over 160 IBD susceptibility genes identified so far [5]), a disruption of the intestinal tolerance occurs towards commensal bacteria. While the carriage of IBD risk alleles alone does not determine disease onset, one or several additional, probably exogenous factors must be present. One key question is if this inappropriate and uncontrolled inflammatory response towards the intestinal microbiome is secondary to an acquired change of the microbial composition. Indeed, in patients with IBD, a marked dysregulation of the coloncolonic and intestinal microbiome, called ‘dysbiosis’, is observed, commonly characterized by a decrease in Firmicutes, an expansion of Proteobacteria, along with a decrease in community richness [6]. Alternatively, could a subtle immune defect occur in one or several control steps, crucial for the maintenance of normal intestinal homeostasis, which subsequently might cause a change of the intestinal bacterial composition? It is possible that both factors could contribute to the onset and maintenance of inflammation: the type of immune responses of the host as well as the composition of the intestinal microbiome, which is the preferred hypothesis of the author.
The type of immune responses of the host as well as the composition of the intestinal microbiome could both contribute to the onset and maintenance of inflammation
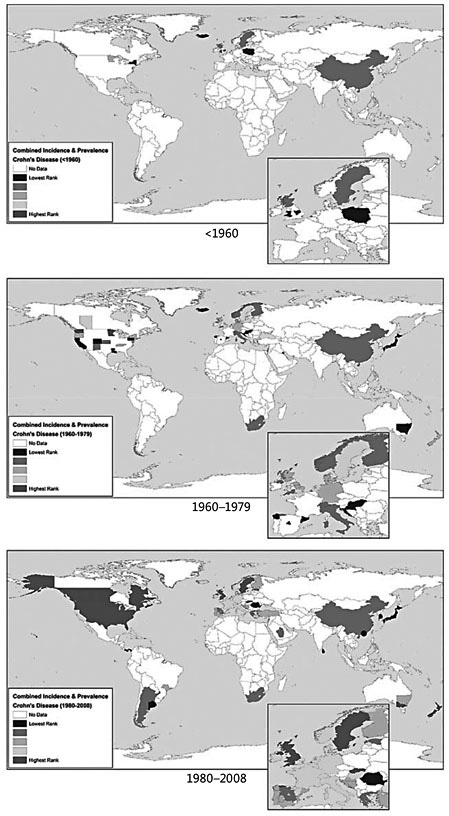
The observation of a marked increase of the incidence of IBD in general and pediatric forms, in particular over the past 50 years ( fig. 1 ), is a major argument favoring environmental changes as crucial trigger factor for the development of IBD. Genetic factors cannot change within such a short time frame, the disease susceptibility remains almost identical over few generations, but the raised IBD incidence in countries with a Western lifestyle clearly points out to a modification of the lifestyle as main driver of the development of IBD [3]. What are the changes that may disrupt the intestinal homeostasis and thus lead to the increase of IBD? Top research addressing this question is currently underway and some aspects will be reviewed in this article.
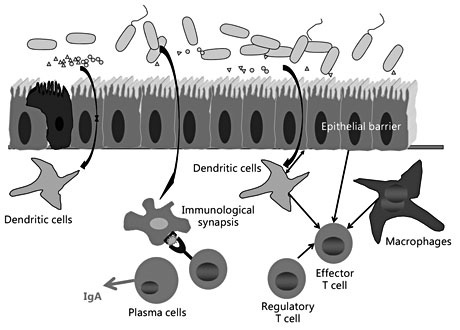
Over the last years, it has become clear that intestinal homeostasis ( fig. 2 ) requires a balanced interaction between the intestinal immune system (with innate and adaptive immune responses) and the microbiome. An intact intestinal epithelial barrier assuring a normal bacterial clearance of the intestinal surface is crucial to guarantee this homeostasis. Any factors affecting the epithelial barrier function directly or indirectly may impact on this homeostasis, as well as any changes of the intestinal microbial composition. It is intriguing to learn that some frequently used food components impact on the quality of the intestinal barrier, as well as on the composition of the intestinal microbiome. This highlights the close interactions between living conditions, hygiene, food habits and food quality with the bacterial composition of the intestinal microbiome and the activation status of the intestinal immune system.
Based on these pathophysiological aspects, it is easily understandable that research recently started to focus on the impact of food on the pathophysiology of IBD, and, perhaps even more important, the role of food or diets in treating IBD [7, 8].
EEN has a strong and rapid anti-inflammatory effect in patients with CD, with reduction of systemic and mucosal inflammatory parameters within a few days of initiation [28]. No randomized controlled trial (RCT) compared EEN to placebo in children with CD. However, two pediatric meta-analyses as well as a Cochrane review (combining pediatric and adult data) analyzed the efficacy of EEN as induction therapy for CD compared to steroids [29–31]. Clinical trials comparing EEN to steroids based on remission rates as outcome parameter showed an overall remission rate of approximately 75% for EEN at the end of exclusive treatment. Remission rates with steroids were not different from EEN-induced remission in these two meta-analyses based on a total of 11 pediatric clinical trials [32–41]. A further pediatric study has recently been published [41], showing data in line with the previous studies. It is somewhat challenging to summarize the efficacy of EEN based on these openlabel studies, since there are major differences in how EEN is performed (duration, feed types, as well as outcome measures) [42, 43]. Two recent large single-center studies, each based on more than 100 pediatric CD patients, further support the results of the clinical trials, each one showing a remission rate of approximately 80% [44, 45] . Six different studies analysed the potential of EEN to induce mucosal healing, with healing rates from 19 to 75% [28, 38, 45–48]. The definition of mucosal healing differs between the studies, making them difficult to compare. The rate of mucosal healing was clearly higher in patients on EEN compared to those with steroid-induced remission [38, 46]. One RCT included mucosal healing as outcome parameter, indicating a clear superiority of 10 weeks of EEN compared to steroids, with mucosal healing rates of 74 and 33% for EEN and steroids, respectively [49].
Data from adult patients are not in keeping with the pediatric studies and rates to induce remission with EEN are lower, probably due to lower adherence to the exclusivity principle and also less experience among adult-IBD experts with the use of this nutritional intervention. The best results for EEN occur in IBD centers that regularly use EEN as treatment option, while remission rates differ significantly in centers that rarely or almost never use EEN.
Different liquid nutritional products are available and were tested in the treatment of CD showing its efficacy in children and adult patients [39–41], as demonstrated by RCTs [27–29, 34]. Efficacy does not depend on the protein source; polymeric or elemental feeds equally induce remission in CD patients, while acceptability as well as costs of EN differ markedly between elemental diets or polymeric feeds: elemental feeds are less often tolerated by mouth and patients often choose nasogastric tube administration. In contrast, for patients receiving polymeric feeds, the oral route is most often the first choice [44]. We have recently demonstrated that there is no significant difference in remission between oral- and continuous nasogastric tube-induced feeds [44]. Therefore, in our center, according to the recent pediatric guidelines, we always offer oral feeds with a polymeric formula, while EEN via a nasogastric tube remains reserved for patients unable to achieve the desired caloric intake or who refuse oral feeding due to taste or texture [50]. Elemental feeds should only be reserved for patients allergic to cow’s milk proteins. As many patients have weight loss and/or growth retardation, the estimated energy requirements are above the recommended intake. Therefore, we most often use 120% or more of the normal caloric requirements adjusted to age and estimated needed catchup growth.
The most important point when using EN as induction therapy is to use it on an exclusive basis, without any additional foods. Johnson et al. [51] showed in a RCT (exclusive EN vs. partial EN with normal diet over 6 weeks using an elemental formula) clear superiority for full EEN over partial EN in remission rates at 6 weeks [10/24 (42%) vs. 4/26 (15%), respectively]. In that study, efficacy of EEN was markedly lower than in most other published studies, and there was a high dropout rate in both arms, highlighting the relevance of compliance. Compliance a priori is not any better in children than in adult patients, but marked growth retardation and the potential of EEN to allow efficient catchup growth is a major motivation for children and adolescents with CD. Close monitoring and regular home visits by a dietician or nurse further improve the successful use of EEN.
Maintenance of Remission in CD
The role of maintenance therapy based on EN is less clear. Several studies analyzed the potential of nutritional supplementation as long-term therapy (alone or in addition to standard therapy). A recent meta-analysis [52] highlighted that overall clinical remission rates were higher in patients with EN than in those without. The quantity of enteral formula used was shown to be important: higher amounts of enteral formula were associated with higher remission rates. However, large RCTs are necessary to assess a definite role of EN for the maintenance of remission.
The mode of action of EN to treat CD is still not completely understood despite many ongoing studies. Several mechanisms have been proposed: reduced allergenic load, being nucleotide free, no addition of food additives, and an anti-inflammatory lipid composition. In line with the mechanisms discussed above, a new hypothesis has recently been developed in that EEN has a specific effect on the intestinal microbiome, positively interfering with the dysbiosis in CD patients. Some studies analyzed changes in the microbiome during and after EEN [53, 54] .
The specific carbohydrate diet (SCD) was introduced and initially described in the early 1920s to treat celiac disease. In the years that followed, it became very popular due to several impressive lay reports indicating the potential of SCD to cure various diseases, including UC. The underlying theory of the SCD is that disaccharide and polysaccharide carbohydrates are poorly absorbed in the human intestinal tract, resulting in bacterial and yeast overgrowth. This might lead to an inflammatory stimulation with mucosal damage, subsequently causing an aggravation of carbohydrate malabsorption and a vicious auto-amplifying cycle. Thus, the recommendation is to restrict carbohydrate exposure to the monosaccharides glucose, fructose, and galactose. However, today significant variations of a carbohydrate-free (reduced) diet exist. For example, a less strict version is a gluten-free diet. These effects are hypothesized to result in small bowel injury, thus perpetuating the cycle of carbohydrate malabsorption and intestinal injury. A very recent retrospective study [55] in 26 children with IBD indicated that the use of a specific carbohydrate-free diet is potentially helpful in maintaining remission, as highlighted by a marked drop in disease activity scores. It is important to mention that most patients remained on maintenance drug therapy and only one half of the patients had a strict SCD.
The FODMAP diet’s rationale is close to that of the SCD in that poorly absorbed carbohydrates (fermentable oligo-, di- and mono-saccharides and polyols, FODMAPs) result in intestinal bacterial overgrowth, a reduced intestinal barrier and secondary mucosal inflammation [56]. However, despite this overlap, there are significant practical differences: the FODMAP diet is highly restrictive of certain fruits and vegetables, while the SCD has unrestricted fruit and vegetable intake except for potatoes and yams. So far, only two studies evaluated the role of FODMAP diet in IBD, 1 in 8 UC patients after colectomy [57], the second was a retrospective study in 72 adult IBD patients [58], indicating a significant effect of reducing abdominal pain, bloating and stool frequency, as long as patients were adherent to the diet. A prospective evaluation of 5 patients after colectomy for UC failed to show any benefit from a reduction of FODMAPs in the diet [57].
An innovative nutritional approach is based on partial EN plus a very strict exclusion diet avoiding animal fat, high sugar intake, gliadin, and consumption of emulsifiers and maltodextrin [7]. In a pilot study of 47 CD patients [59] (34 children and 13 adult patients), excellent response and remission rates were obtained (79 and 70%, respectively). Normalization of previously elevated Creactive protein occurred in 70% of the patients who achieved remission. A RCT that further elaborates on the role of this CD exclusion diet as a new treatment option is underway.
Another approach is the so-called Paleo diet, which was rediscovered from a publication in the New England Journal of Medicine in 1985 [60]. This diet is based on an evolutionary hypothesis that the human digestive tract is insufficiently evolved to handle foods resulting from modern agricultural techniques. The exposure of the human digestive tract to foods that were not present at the time of human evolution may result in modern diseases. The Paleo diet privileges the intake of lean, non-domesticated meats and non-cereal plant-based foods (i.e. fruits, roots, legumes, and nuts). So far, no data on the role of the Paleo diet in the treatment of IBD exist.
One important flipside of these diets, besides their very restrictive character, is the potential of creating deficiency of particular nutrients, especially vitamins. Both the SCD and the Paleo diet have the potential to cause vitamin D deficiency. This is a particular concern, given the importance of sufficient vitamin D levels for normal immune function, particularly in the gastrointestinal tract. Vitamin D deficiency was recently associated with an increased risk of surgery and hospitalization in IBD patients [61].
An internet search of dietary recommendations for patients with CD indicated that references to vegetables, fruits, and fibers were particularly common. For instance, avoidance of fatty and fried foods was unanimously indicated for CD and in over 70% of sites specifically for UC [62]. Avoidance of raw vegetables, cruciferous vegetables, citrus fruit, red meat, carbonated beverages, coffee and tea, alcohol, fatty and fried foods, spicy foods, sugars, seeds, and popcorn were also often mentioned, while an increased intake of cooked vegetables, fish, poultry, lean protein, and a high-protein diet were also frequently recommended. The authors concluded that ‘our web search analysis demonstrated that patient-targeted dietary recommendations are highly restrictive and frequently conflicting. These recommendations may result in patient confusion and unnecessarily restrictive diets’. This analysis nicely summarized the desperate search of many patients for dietary/nutritional interventions for CD and UC. This is a promising concept, but it has to be based on the pathophysiological aspects of the disease, rather than ideological believing and nutritional recommendations. It has to be based on well-designed and performed clinical trials. It is encouraging that several trials are planned or already underway telling us in the near future what might be the most efficient way to treat our patients with IBD, presumably in a personalized way combining nutritional and medical strategies.
The writing of this article was supported by Nestlé Nutrition Institute.
- Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM: Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis 2011; 17: 423–439.
- Auvin S, Molinie F, Gower-Rousseau C, Brazier F, Merle V, Grandbastien B, et al: Incidence, clinical presentation and location at diagnosis of pediatric inflammatory bowel disease: a prospective population-based study in northern France (1988–1999). J Pediatr Gastroenterol Nutr 2005; 41: 49–55.
- Ruemmele FM: Pediatric inflammatory bowel diseases: coming of age. Curr Opin Gastroenterol 2010; 26: 332–336.
- Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, et al: The ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr 2014; 58: 795–806.
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al: Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012; 491: 119–124.
- Nagalingam NA, Lynch SV: Role of the microbiota in inflammatory bowel diseases. Inflamm Bowel Dis 2012; 18: 968–984.
- Pfeffer-Gik T, Levine A: Dietary clues to the pathogenesis of Crohn’s disease. Dig Dis 2014; 32: 389–394.
- Ruemmele FM, Garnier-Lengline H: Transforming growth factor and intestinal inflammation: the role of nutrition. Nestle Nutr Inst Workshop Ser 2013; 77: 91–98.
- Amre DK, D’Souza S, Morgan K, Seidman G, Lambrette P, Grimard G, et al: Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn’s disease in children. Am J Gastroenterol 2007; 102: 2016–2025.
- Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault MC, Carbonnel F: Animal protein intake and risk of inflammatory bowel disease: the E3N prospective study. Am J Gastroenterol 2010; 105: 2195–2201.
- Shoda R, Matsueda K, Yamato S, Umeda N: Epidemiologic analysis of Crohn disease in Japan: increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. Am J Clin Nutr 1996; 63: 741–745.
- Hart AR, Luben R, Olsen A, Tjonneland A, Linseisen J, Nagel G, et al: Diet in the aetiology of ulcerative colitis: a European prospective cohort study. Digestion 2008; 77: 57–64.
- Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Fuchs CS, et al: Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut 2014; 63: 776–784.
- John S, Luben R, Shrestha SS, Welch A, Khaw KT, Hart AR: Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: a UK prospective cohort study. Eur J Gastroenterol Hepatol 2010; 22: 602–606.
- Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, et al: A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 2013; 145: 970–977.
- Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA: Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol 2008; 6: 121–131.
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al: Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 2010; 107: 14691–14696.
- Nickerson KP, Homer CR, Kessler SP, Dixon LJ, Kabi A, Gordon IO, et al: The dietary polysaccharide maltodextrin promotes Salmonella survival and mucosal colonization in mice. PLoS One 2014; 9:e101789.
- McDonald DE, Pethick DW, Mullan BP, Hampson DJ: Increasing viscosity of the intestinal contents alters small intestinal structure and intestinal growth, and stimulates proliferation of enterotoxigenic Escherichia coli in newly-weaned pigs. Br J Nutr 2001; 86: 487–498.
- Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, et al: High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 2004; 127: 412– 421.
- Nickerson KP, McDonald C: Crohn’s disease- associated adherent-invasive Escherichia coli adhesion is enhanced by exposure to the ubiquitous dietary polysaccharide maltodextrin. PLoS One 2012; 7:e52132.
- Moyana TN, Lalonde JM: Carrageenan-induced intestinal injury in the rat – a model for inflammatory bowel disease. Ann Clin Lab Sci 1990; 20: 420–426.
- Swidsinski A, Ung V, Sydora BC, Loening- Baucke V, Doerffel Y, Verstraelen H, et al: Bacterial overgrowth and inflammation of small intestine after carboxymethyl cellulose ingestion in genetically susceptible mice. Inflamm Bowel Dis 2009; 15: 359–364.
- Swidsinski A, Sydora BC, Doerffel Y, Loening- Baucke V, Vaneechoutte M, Lupicki M, et al: Viscosity gradient within the mucus layer determines the mucosal barrier function and the spatial organization of the intestinal microbiota. Inflamm Bowel Dis 2007; 13: 963–970.
- Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R: A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990; 98: 694–702.
- Ruemmele FM, Roy CC, Levy E, Seidman EG: Nutrition as primary therapy in pediatric Crohn’s disease: fact or fantasy? J Pediatr 2000; 136: 285–291.
- Lochs H, Dejong C, Hammarqvist F, Hebuterne X, Leon-Sanz M, Schutz T, et al: ESPEN Guidelines on enteral nutrition: gastroenterology. Clin Nutr 2006; 25: 260–274.
- Fell JM, Paintin M, Arnaud-Battandier F, Beattie RM, Hollis A, Kitching P, et al: Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment Pharmacol Ther 2000; 14: 281–289.
- Heuschkel RB, Menache CC, Megerian JT, Baird AE: Enteral nutrition and corticosteroids in the treatment of acute Crohn’s disease in children. J Pediatr Gastroenterol Nutr 2000; 31: 8–15.
- Zachos M, Tondeur M, Griffiths AM: Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2007;Cd000542.
- Dziechciarz P, Horvath A, Shamir R, Szajewska H: Meta-analysis: enteral nutrition in active Crohn’s disease in children. Aliment Pharmacol Ther 2007; 26: 795–806.
- Seidman E: Semi-elemental (S-E) diet versus prednisone in pediatric Crohn’s disease. Gastroenterology 1994; 104:A778.
- Seidman E: Elemental diet versus prednisone as initial therapy in Crohn’s disease: early and long term results. Gastroenterology 1991; 100:A250.
- Sanderson IR, Udeen S, Davies PS, Savage MO, Walker-Smith JA: Remission induced by an elemental diet in small bowel Crohn’s disease. Arch Dis Child 1987; 62: 123–127.
- Thomas AG, Taylor F, Miller V: Dietary intake and nutritional treatment in childhood Crohn’s disease. J Pediatr Gastroenterol Nutr 1993; 17: 75–81.
- Griffiths A: Elemental versus polymeric enteral nutrition as primary therapy for active Crohn’s disease: a multi-centre pediatric randomized controlled trial. J Pediatr Gastroenterol Nutr 2000;31:S75 .
- Terrin G CR, Ambrosini A, et al: A semielemental diet (Pregomin) as primary therapy for inducing remission in children with active Crohn’s disease. Ital J Pediatr 2002; 28: 401–405.
- Borrelli O, Cordischi L, Cirulli M, Paganelli M, Labalestra V, Uccini S, et al: Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol 2006; 4: 744–753.
- Akobeng AK, Miller V, Stanton J, Elbadri AM, Thomas AG: Double-blind randomized controlled trial of glutamine-enriched polymeric diet in the treatment of active Crohn’s disease. J Pediatr Gastroenterol Nutr 2000; 30: 78–84.
- Ludvigsson JF, Krantz M, Bodin L, Stenhammar L, Lindquist B: Elemental versus polymeric enteral nutrition in paediatric Crohn’s disease: a multicentre randomized controlled trial. Acta Paediatr 2004; 93: 327–335.
- Grogan JL, Casson DH, Terry A, Burdge GC, El-Matary W, Dalzell AM: Enteral feeding therapy for newly diagnosed pediatric Crohn’s disease: a double-blind randomized controlled trial with two years follow-up. Inflamm Bowel Dis 2012; 18: 246–253.
- Day AS, Whitten KE, Sidler M, Lemberg DA: Systematic review: nutritional therapy in paediatric Crohn’s disease. Aliment Pharmacol Ther 2008; 27: 293–307.
- Wilson DC, Thomas AG, Croft NM, Newby E, Akobeng AK, Sawczenko A, et al: Systematic review of the evidence base for the medical treatment of paediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2010; 50(suppl 1):S14–S34.
- Rubio A, Pigneur B, Garnier-Lengline H, Talbotec C, Schmitz J, Canioni D, et al: The efficacy of exclusive nutritional therapy in paediatric Crohn’s disease, comparing fractionated oral vs. continuous enteral feeding. Aliment Pharmacol Ther 2011; 33: 1332–1339.
- Buchanan E, Gaunt WW, Cardigan T, Garrick V, McGrogan P, Russell RK: The use of exclusive enteral nutrition for induction of remission in children with Crohn’s disease demonstrates that disease phenotype does not influence clinical remission. Aliment Pharmacol Ther 2009; 30: 501–507.
- Berni Canani R, Terrin G, Borrelli O, Romano MT, Manguso F, Coruzzo A, et al: Shortand long-term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn’s disease. Dig Liver Dis 2006; 38: 381–387.
- Afzal NA, Van Der Zaag-Loonen HJ, Arnaud- Battandier F, Davies S, Murch S, Derkx B, et al: Improvement in quality of life of children with acute Crohn’s disease does not parallel mucosal healing after treatment with exclusive enteral nutrition. Aliment Pharmacol Ther 2004; 20: 167–172.
- Beattie RM, Schiffrin EJ, Donnet-Hughes A, Huggett AC, Domizio P, MacDonald TT, et al: Polymeric nutrition as the primary therapy in children with small bowel Crohn’s disease. Aliment Pharmacol Ther 1994; 8: 609– 615.
- Borrelli O, Bascietto C, Viola F, Bueno de Mesquita M, Barbato M, Mancini V, et al: Infliximab heals intestinal inflammatory lesions and restores growth in children with Crohn’s disease. Dig Liver Dis 2004; 36: 342– 347.
- Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, et al: Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis 2014; 8: 1179–1207.
- Johnson T, Macdonald S, Hill SM, Thomas A, Murphy MS: Treatment of active Crohn’s disease in children using partial enteral nutrition with liquid formula: a randomised controlled trial. Gut 2006; 55: 356–361.
- Yamamoto T, Nakahigashi M, Umegae S, Matsumoto K: Enteral nutrition for the maintenance of remission in Crohn’s disease: a systematic review. Eur J Gastroenterol Hepatol 2010; 22: 1–8.
- Lionetti P, Callegari ML, Ferrari S, Cavicchi MC, Pozzi E, de Martino M, et al: Enteral nutrition and microflora in pediatric Crohn’s disease. JPEN J Parenter Enteral Nutr 2005; 29 (suppl 4):S173–S175; discussion S5–S8, S84–S88.
- Shiga H, Kajiura T, Shinozaki J, Takagi S, Kinouchi Y, Takahashi S, et al: Changes of faecal microbiota in patients with Crohn’s disease treated with an elemental diet and total parenteral nutrition. Dig Liver Dis 2012; 44: 736–742.
- Obih C, Wahbeh G, Lee D, Braly K, Giefer M, Shaffer ML, et al: Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition 2016; 32: 418–425.
- Gibson PR, Shepherd SJ: Personal view: food for thought – western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment Pharmacol Ther 2005; 21: 1399–1409.
- Croagh C, Shepherd SJ, Berryman M, Muir JG, Gibson PR: Pilot study on the effect of reducing dietary FODMAP intake on bowel function in patients without a colon. Inflamm Bowel Dis 2007; 13: 1522–1528.
- Gearry RB, Irving PM, Barrett JS, Nathan DM, Shepherd SJ, Gibson PR: Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease-a pilot study. J Crohns Colitis 2009; 3: 8–14.
- Sigall-Boneh R, Pfeffer-Gik T, Segal I, Zangen T, Boaz M, Levine A: Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm Bowel Dis 2014; 20: 1353–1360.
- Eaton SB, Konner M: Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med 1985; 312: 283–289.
- Ananthakrishnan AN, Cagan A, Gainer VS, Cai T, Cheng SC, Savova G, et al: Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn’s disease. Inflamm Bowel Dis 2013; 19: 1921–1927.
- Hou JK, Lee D, Lewis J: Diet and inflammatory bowel disease: review of patient-targeted recommendations. Clin Gastroenterol Hepatol 2014; 12: 1592–1600.
- Molodecky NA, et al: Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46–52 .